Building Regional Filtration Supply Chains
The New Regional Model to Ensure Consistent Supply of High-Quality Filters for Biomanufacturing
We’re investing in manufacturing locally with built-in redundancy, ensuring business continuity and reducing supply risks for your filtration needs.
With increasing volatility in global supply chains, you need a reliable, consistent, and secure filter supply. To deliver on that, we’ve reimagined our filter manufacturing supply chains.
We’re building a hybrid global-local filter manufacturing network—combining global oversight and regional management—to minimize the impact of disruptive forces on your operations, ensure business continuity, and give you peace of mind.
Stay ahead by subscribing to our newsletter, which includes updates on our supply network, new products and services, and best practices.
Global Design. Local Execution
We’ve expanded our manufacturing footprint with a new filter manufacturing site in Blarney, Ireland, a new membrane manufacturing site in Darmstadt, Germany, and increased membrane manufacturing capacity in Cork, Ireland to meet the needs of the Europe, Middle East, and Africa region. This helps us build a regional supply model that brings manufacturing closer to your operations, which results in:
- Increased production capacity for filters and membranes
- Dedicated regionalized capabilities
- Minimized risk for supply disruptions
- Streamlined logistics and shorter transportation distances
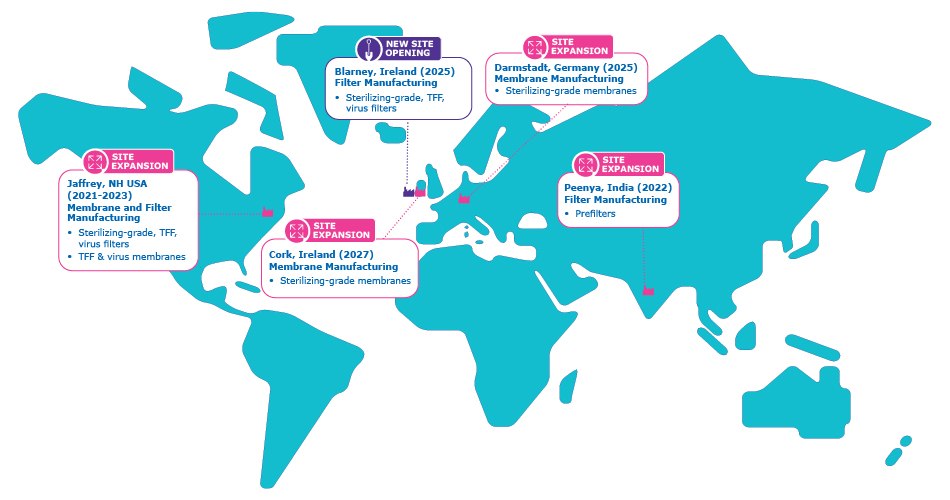
Map 1:Our Filter Manufacturing Footprint for Drug Development and Manufacturing.
Same Products, Same Standards Across Our Manufacturing Network
In biomanufacturing, switching suppliers consumes your time and resources to verify no impacts to your product quality. That's why all our filters will be released to the same global quality standards with no changes to product specifications or part numbers, irrespective of geography.
Regardless of which of our sites you order from, you can expect:
- Consistent product performance and same specifications, backed up by full validation documentation
- Lot-to-lot consistency
- Emprove® dossiers with comprehensive documentation to facilitate your qualification, risk assessment, and process optimization, available for a selection of filters.
We provide validation documentation, change notifications, and audit support to facilitate dual-site validation and ensure supply chain continuity—giving you peace of mind, especially in critical or time-sensitive applications.
Lower Emissions, Same High Standards
Sustainability isn’t an extra—it’s embedded in how we build and operate:
- Our new Blarney facility is climate neutral
- Signed virtual power purchase agreements for European operations
- 100% renewable electricity in USA since 2022
- Peenya, India site is a LEED gold-certified facility
- We continuously work towards energy and resource efficiency at our sites globally.
Our commitment to sustainability includes our bulk packaging. In our Millistak+® HC Pro line, for example, our streamlined packaging has resulted in a:
- 42% average reduction in corrugated packaging material per product
- 29% decrease in the number of pallets, further reducing related emissions
- 75% reduction in operator time to open and manage the product and packaging
Supply Chain Resilience and Robustness Are Built-in
Our manufacturing network has more than new facilities – we built in resilience and robustness.
With our regional and redundant network of filter manufacturing sites, we ensure a reliable filter supply even when global events create local disruptions. All our sites are ISO 9001: 2015 certified and covered by business continuity plans. Our products are released to the same high-quality standards, ensuring consistent performance, no matter where they are manufactured, thanks to global quality governance and globally harmonized sourcing.
Want to be prepared to handle future disruption? Our dual-site validation readiness means you can switch sites without risking downtime. We provide:
- Full validation documentation
- Prompt change notifications
- Transparent risk assessments and business continuity plans
We’re also investing in supply chain digitization through our eMERGE™ program, giving you access to harmonized quality supplier data, predictive analytics, and faster issue resolution.
You can be assured of seamless site qualification with our comprehensive notification plan, summary reports, audit support and validation assistance.
Explore some of our globally manufactured filtration products:
- Sterilizing-grade filters: Our broad portfolio of Durapore® and Millipore Express® membranes offers options for efficient processing of every pharma and biopharma stream; from cell culture media through process intermediates to final sterile filtration. Our membrane filters offer the highest level of sterility assurance, are available in different formats, and are supported by comprehensive documentation packages to meet your compliance needs.
- Tangential flow filtration devices: From single-use to multi-use, Pellicon® filters are ideal for high value mAb, ADC, plasma IgG, vaccine, and viral vector applications. Reliably delivering purity and consistent performance at every stage and scale, throughout the lifecycle of your drug products.
- Virus filters: The Viresolve® Pro solution provides a comprehensive and flexible template for viral clearance in downstream processing of both monoclonal antibodies (mAbs) and recombinant proteins. This proven virus filtration solution delivers the highest levels of virus retention and productivity across a broad range of operating conditions.
Experience the benefits of our filters yourself: Request your free sample today.
Related Products
To continue reading please sign in or create an account.
Don't Have An Account?