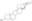239803 Sigma-AldrichCyclopamine, V. californicum - CAS 4449-51-8 - Calbiochem
Cyclopamine, V. californicum, CAS 4449-51-8, is a cell-permeable steroidal alkaloid & cholesterol mimic that specifically antagonizes Sonic Hedgehog signaling through direct interaction with Smo.
More>> Cyclopamine, V. californicum, CAS 4449-51-8, is a cell-permeable steroidal alkaloid & cholesterol mimic that specifically antagonizes Sonic Hedgehog signaling through direct interaction with Smo. Less<<Synonymes: Shh Signaling Antagonist I
Produits recommandés
Aperçu
| Replacement Information |
|---|
Tableau de caractéristiques principal
| CAS # | Empirical Formula |
|---|---|
| 4449-51-8 | C₂₇H₄₁NO₂ |
Prix & Disponibilité
| Référence | Disponibilité | Conditionnement | Qté | Prix | Quantité | |
|---|---|---|---|---|---|---|
| 239803-1MG |
|
Flacon en verre | 1 mg |
|
— | |
| 239803-5MG |
|
Flacon en verre | 5 mg |
|
— |
| Description | |
|---|---|
| Overview | A cell-permeable steroidal alkaloid and cholesterol mimic that displays both teratogenic and antitumor activities. It disrupts cholesterol bio-synthesis and specifically antagonizes shh (Sonic Hedgehog) signaling pathway through direct interaction with Smo (smoothened), a distant relative of G-protein-coupled receptors. A valuable tool for studying the involvement of shh signaling in the development of various tumors. Tomatidine (Cat. No. 614350) can be used as a negative control. Also available as a 10 mM solution in Ethanol (Cat. No. 239806). |
| Catalogue Number | 239803 |
| Brand Family | Calbiochem® |
| Synonyms | Shh Signaling Antagonist I |
| Product Information | |
|---|---|
| CAS number | 4449-51-8 |
| ATP Competitive | N |
| Form | Off-white solid |
| Hill Formula | C₂₇H₄₁NO₂ |
| Chemical formula | C₂₇H₄₁NO₂ |
| HS Code | 2937 29 90 |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | Hh signaling in Shh-light2 assay |
| Primary Target IC<sub>50</sub> | 20 nM |
| Purity | ≥97% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS | |
|---|---|
| RTECS | GY0750000 |
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Référence | GTIN |
| 239803-1MG | 04055977198867 |
| 239803-5MG | 04055977198874 |
Documentation
Cyclopamine, V. californicum - CAS 4449-51-8 - Calbiochem FDS
| Titre |
|---|
Cyclopamine, V. californicum - CAS 4449-51-8 - Calbiochem Certificats d'analyse
| Titre | Numéro de lot |
|---|---|
| 239803 |
Références bibliographiques
| Aperçu de la référence bibliographique |
|---|
| Berman, D.M., et al. 2003. Nature, 425, 846. Thayer, S.P., et al. 2003. Nature, 425, 851. Watkins, D.N., et al. 2003. Nature 422, 313. Chen, J.K., et al. 2002. Proc. Natl. Acad. Sci. USA 99, 14071. Berman, D.M., et al. 2002. Science 297, 1559. Taipale, J., et al. 2000. Nature 406, 1005. Kim, S.K., and Melton, D.A. 1998. Proc. Natl. Acad. Sci. USA 95, 13036. Cooper, M.K., et al. 1998. Science 280, 1603. Incardona, J.P., et al. 1998. Development 125, 3553. |