375645 Sigma-AldrichHePTP Inhibitor, ML119 - CAS 357302-57-9 - Calbiochem
The HePTP Inhibitor, ML119 controls the biological activity of HePTP. This small molecule/inhibitor is primarily used for Phosphorylation & Dephosphorylation applications.
More>> The HePTP Inhibitor, ML119 controls the biological activity of HePTP. This small molecule/inhibitor is primarily used for Phosphorylation & Dephosphorylation applications. Less<<Sinonimi: (Z)-2-(5-(7-Bromo-3-oxo[1,3]thiazolo(2ʹ,3ʹ:2,3)imidazo[4,5-b]pyridin-2(3H)-ylidene)methyl)furan-2-yl)benzoic acid, PTPN7 Inhibitor, ML119, CID1357397, CD45 Inhibitor IV, Dusp6/MKP3 Inhibitor II, EC-PTP/PCPTP1 Inhibitor II, Lyp Inhibitor II, PTP Inhibitor XXX, PTP-SL Inhibitor II, PTP1B Inhibitor VIII, PTPN2/TCPTP Inhibitor III, SHP1 Inhibitor VIII, SHP2 Inhibitor V, STEP Inhibitor, VHR Inhibitor III
Prodotti consigliati
Panoramica
| Replacement Information |
|---|
Tabella delle specifiche principali
| CAS # | Empirical Formula |
|---|---|
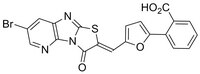
| 357302-57-9 | C₂₀H₁₀BrN₃O₄S |
Prezzi e disponibilità
| Numero di catalogo | Disponibilità | Confezionamento | Qtà/conf | Prezzo | Quantità | |
|---|---|---|---|---|---|---|
| 375645-10MG |
|
Bottiglia di vetro | 10 mg |
|
— |
| References | |
|---|---|
| References | Sergienko, E., et al. 2012. ACS Chem. Biol. 7, 367. |
| Product Information | |
|---|---|
| CAS number | 357302-57-9 |
| Form | Greenish-yellow solid |
| Hill Formula | C₂₀H₁₀BrN₃O₄S |
| Reversible | Y |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | HePTP/PTPN7 |
| Primary Target IC<sub>50</sub> | 210 nM |
| Primary Target K<sub>i</sub> | 211 nM for HePTP |
| Secondary target | other phosphatases |
| Purity | ≥98% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Numero di catalogo | GTIN |
| 375645-10MG | 04055977212921 |
Documentation
HePTP Inhibitor, ML119 - CAS 357302-57-9 - Calbiochem MSDS
| Titolo |
|---|
HePTP Inhibitor, ML119 - CAS 357302-57-9 - Calbiochem Certificati d'Analisi
| Titolo | Numero di lotto |
|---|---|
| 375645 |
Riferimenti bibliografici
| Panoramica delle referenze |
|---|
| Sergienko, E., et al. 2012. ACS Chem. Biol. 7, 367. |











 Antibody[209309-ALL].jpg)