Ylide-functionalized Phosphines (YPhos)
Professor Viktoria Gessner and coworkers from the Ruhr-University Bochum have developed a class of ylide-substituted phosphine ligands (YPhos) that contain a bulky ylide-substituent directly bound at the phosphorus atom.1 These electron-rich phosphines facilitate palladium-catalyzed coupling reactions at remarkably mild reaction conditions. They enable the conversion of often challenging aryl chlorides in good yield with short reaction times. For example, keYPhosTM and joYPhosTM perform excellently in the Buchwald-Hartwig aminations at room temperature. joYPhos™ also readily converts aryl halides (including chlorides) with organolithium, Grignard, and zinc reagents. The strongest donor, trYPhosTM , is best suited for challenging alpha-arylations.
Key Advantages of YPhos Ligands for Palladium-Catalyzed Coupling Reactions
- Facile coupling of aryl halides, especially aryl chlorides
- Mild reaction conditions
- High activity in C-N and C-C cross-coupling reactions
- Coupling of organolithium, magnesium and zinc reagents
Applications of YPhos Ligands in C–N and C–C Coupling of Aryl Halides
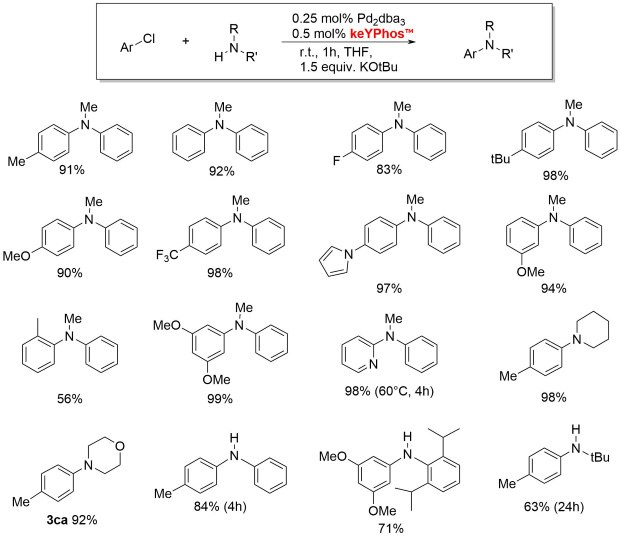
Figure 2.keYPhos™ with a methyl group in the ylide-backbone is a valuable ligand for the palladium-catalyzed coupling of aryl chlorides with primary and secondary alkyl and aryl amines at room temperature. The ligand performs well with common palladium sources such as Pd2(dba)3, Pd(OAc)2, [Pd(allyl)Cl]2 or [Pd(cinamyl)Cl]2.2,3
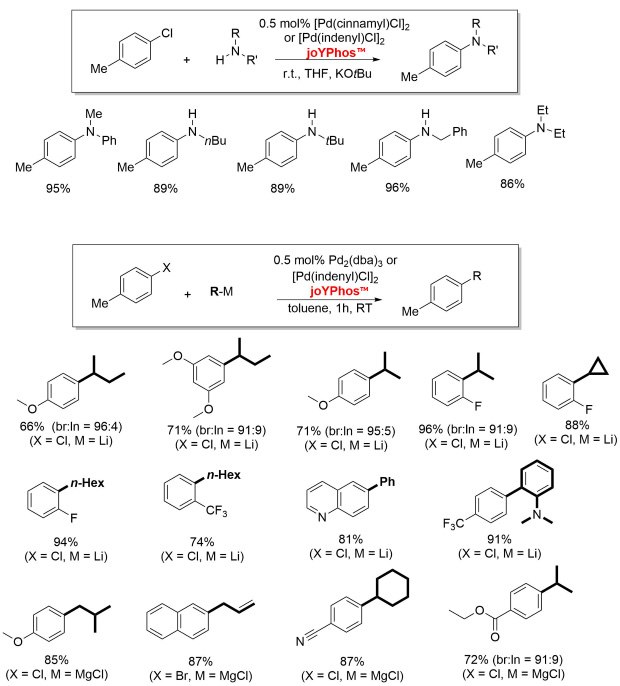
Figure 3.JoYPhos™ featuring a phenyl group at the ylide substituent is also an excellent ligand for Buchwald-Hartwig aminations4,5 as well as for C-C coupling reactions. It allows the previously unprecedented direct coupling of alkyllithium reagents with aryl chloride.6
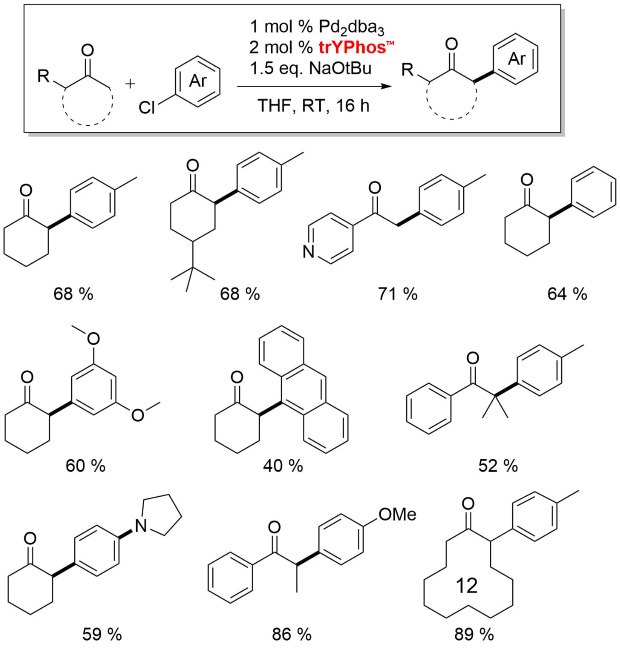
Figure 4.In this series, trYPhos™ is the most electron-rich YPhos ligand and was found to be active for alpha-arylation of aliphatic cyclic ketones.7
References
To continue reading please sign in or create an account.
Don't Have An Account?