Buchwald Catalysts & Ligands
As a chemist, you're focused on discovering new chemistry and its practical applications. We support your breakthroughs with an extensive portfolio of Buchwald catalysts and ligands. In collaboration with Stephen Buchwald and his MIT research group, we offer highly active palladium precatalysts and biaryl phosphine ligands for efficient cross-coupling reactions, forming bonds like C-C, C-N, and more. These electron-rich, tunable ligands provide stable, reactive catalyst systems, reducing catalyst loadings, reaction times, and eliminating the need for reducing agents, enabling new methods not achievable with traditional Pd sources.
Products
Products
Buchwald Ligands
- Buchwald and his team developed biaryl phosphine ligands that enhance the reaction’s efficiency, selectivity, and versatility.
- Earlier ligands like dicyclohexyl phosphine dimethylamine enabled aryl chloride–alkyl amine couplings.
- Advanced ligands (e.g. XPhos, di-t-BuXPhos) offer higher activity and compatibility with hindered substrates.
- Bulky ligands enhanced catalytic performance and conversion rates in C–N couplings.
- The Buchwald group also developed copper-catalyzed N-arylation for imidazoles and other heterocycles.
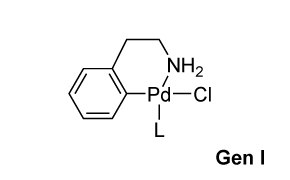
Buchwald Gen 1 Precatalysts
- First-generation (G1) precatalysts employ 2-phenylethan-1-amine–based ligands for enhanced stability in both solution and solid phases.
- Air- and moisture-stable, enabling convenient handling.
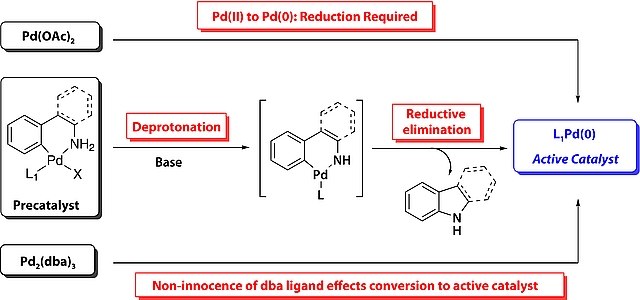
- LPd(0) forms in situ via base-promoted reductive elimination, yielding indoline as a byproduct.
- Higher temperatures are often used with weaker bases to facilitate ligand deprotonation and activate the catalyst.
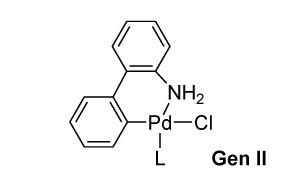
Buchwald Gen 2 Precatalysts
- Second-generation (G2) precatalysts use 2-aminobiphenyl instead of 2-phenylethan-1-amine.
- Shows improved reactivity with weaker bases (e.g., phosphates, weak carbonates).
- Often enable catalyst activation at lower temperatures.
- Enhanced reactivity arises from the greater acidity of the aromatic amine in G2 vs. the aliphatic amine in G1.
- Key features: air and moisture stable, high efficiency, mild conditions, short reaction times, and low catalyst loadings.
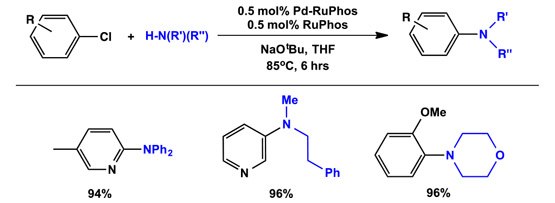
- Demonstrated excellent performance in Suzuki cross-coupling reactions.
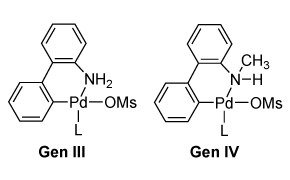
Buchwald Gen 3 and 4 Precatalysts
- G3 and G4 Buchwald precatalysts are advanced, stable palladium complexes for diverse cross-coupling reactions (C–C, C–N, C–O, C–F, C–CF₃, C–S).
- Offer good solubility and activity, which can allow the use of lower catalyst loadings and/or shorter reaction times.
- G3 uses a mesylate ligand (vs. chloride) to enhance solubility, stability, and accommodate bulkier ligands.
- G4 features N-methyl-2-aminobiphenyl as the amine ligand, generating a more benign byproduct (N-methyl carbazole vs carbazole).
- Both generations are effective in key reactions like Suzuki-Miyaura, aminocarbonylation, and N-arylation.
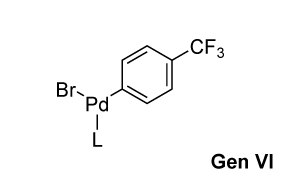
Buchwald Gen 6 Precatalysts
- G6 precatalysts are oxidative addition complexes (OACs), offering advanced performance. These on-cycle precatalysts facilitate easier catalyst activation.
- Retain thermal and air stability while enabling base-free activation and simplified synthesis.
- Avoid generation of carbazole byproducts during precatalyst activation.
- Can support extremely bulky ligands and improve solubility and stability.
- Enable efficient formation of C–C, C–N, C–O, C–F, and C–S bonds.
- Consistently deliver higher reactivity and better yields than earlier generations.
Related Resources
- Brochure: Buchwald Precatalysts
Discover our comprehensive selection of G1 to G6 Buchwald precatalysts, featuring Pd(II) complexes with highly active, versatile, and tunable biarylphosphine ligands.
- Article: G6 Buchwald Precatalysts
Buchwald G6 precatalysts are oxidative addition complexes that enhance palladium-catalyzed cross-coupling reactions. They provide higher reactivity, stability, and simplified synthesis, facilitating the formation of various bonds with improved selectivity and fewer byproducts.
- Article: G3 and G4 Buchwald Precatalysts
G3 and G4 Buchwald palladium precatalysts are the newest air, moisture, and thermally stable crossing-coupling complexes used in bond formation for their versatility and high reactivity.
- Article: G2 Buchwald Precatalysts
Second-generation Buchwald precatalysts, particularly XPhos, improve reactivity in palladium-catalyzed cross-coupling reactions. These catalysts utilize bulky dialkylbiaryl phosphine ligands for enhanced efficiency and stability in synthetic applications.
- Article: Buchwald Phosphine Ligands
Buchwald phosphine ligands for C-C, C-N, and C-O bond formation.
- Article: Buchwald Ligands
Buchwald and coworkers develop versatile phosphine ligands for Pd-catalyzed C–N bond formation; enhancing synthetic reactions for 20 years.
- Article: AlPhos and [(AlPhosPd)2•COD] for Pd-Catalyzed Fluorination
Fluorine containing aromatics (ArF) are desirable compounds with applications in medicinal chemistry and the agricultural industry.
- Buchwald Group – Professor Product Portal
Professor Stephen Buckwald and the Buchwald group have developed a series of highly active and versatile palladium precatalysts and biarylphosphine ligands used in a variety of cross-coupling reactions.
- Article: Scale-Up Guide: Buchwald-Hartwig Amination
Kitalysis high-throughput screening kits are designed for Buchwald-Hartwig amination reactions. These kits contain air and moisture-stable preformed catalysts, facilitating efficient scale-up of catalytic reactions.
References
To continue reading please sign in or create an account.
Don't Have An Account?