Ultrapure Water to Assess Elemental Impurities per USP
This article demonstrates the suitability of fresh ultrapure water produced by a Milli-Q® water purification system for use in ICP-MS trace element analyses according to US Pharmacopeia (USP) General Chapters <231>, <232>, and <233>.
Section Overview
- Elemental Impurities in Pharmaceutical Products
- USP Standards for Purified Water
- Water Quality for ICP-MS Trace Element Analysis
- Trace Elemental Analysis of Milli-Q® Ultrapure Water
- Elemental Impurity Testing: Experimental Conditions and Methods
- Conclusion: Suitability of Milli-Q® Ultrapure Water for Trace Analysis
- Explore Water Systems
Elemental Impurities in Pharmaceutical Products
It is crucial to monitor and control inorganic impurities in pharmaceuticals because some metals are used as reagents and catalysts during production and formulation processes. Moreover, metal impurities can be introduced into pharmaceutical products non-intentionally through contaminated reagents, or when products are in contact with pharmaceutical packaging or with metal surfaces during the development process. Hence, the United States Food and Drug Administration (FDA) and similar international health agencies have had long-standing regulations in place for controlling harmful impurities in pharmaceutical products marketed for human consumption. Historically, four heavy metals (arsenic, cadmium, lead and mercury), or the “Big Four”, were required to be tested according to the United States Pharmacopeial Convention (USP) General Chapter <231> “Heavy Metal Limit Test”. However, new mandatory guidelines to control potential toxic impurities in drugs were recently established in two USP General Chapters <232>1 and <233>.2
These recent chapters in the USP subdivide the metals desired for analyses into several groups. In this article, the class 1 and class 2 impurities comprising 15 metals (cadmium, lead, arsenic, mercury, iridium, osmium, palladium, platinum, rhodium, ruthenium, chromium, molybdenum, nickel, vanadium and copper) are addressed. Other elemental impurities listed in the FDA’s Guidance for Industry to Q3D Elemental Impurities3 and in the European Medicine Agency’s (EMA) Guideline on the Specification Limits for Residues of Metal Catalysts or Metal Reagents4 are addressed in an extended ICP-MS article for pharma.
USP Requirements for Purified Water
The US Pharmacopeia (USP) sets quality levels for purified water to be used by pharmaceutical manufacturers in order to ensure the quality and safety of drugs released on the market. The USP defines acceptable limits for various impurities, including microbial contamination, heavy metals, and chemical residues. Compliance with pharmacopeial standards is crucial for maintaining the integrity of pharmaceutical products, as purified water serves as a key ingredient in formulations, diluents, and cleaning processes. By complying with these recommendations, pharmaceutical and biotech companies can ensure that their products are safe and effective, ultimately protecting patient health.
Water Quality for ICP-MS Trace Element Analysis
Inductively coupled plasma–mass spectrometry (ICP-MS) is a sensitive technique for analyses of elemental impurities.2 Ultrapure water is a main reagent in ICP-MS and is used extensively from sample preparation to analytical instrument cleaning (Figure 1). Milli-Q® ultrapure water purification systems are designed to deliver water that meets or exceeds the water quality standards described in various pharmacopeias. However, water selected as a reagent must not only comply with specific pharmacopeial standards, but must also meet the requirements of modern instrumentation to ensure the best performance of ICP-MS instrumentation.5 Thus, reagent water of very high quality must be selected to avoid sample and analytical instrument contamination and must be free of the elements being measured.6 The aim of this study was to evaluate the suitability of fresh ultrapure water produced using Milli-Q® ultrapure water purification systems for use in ICP-MS trace element analyses.
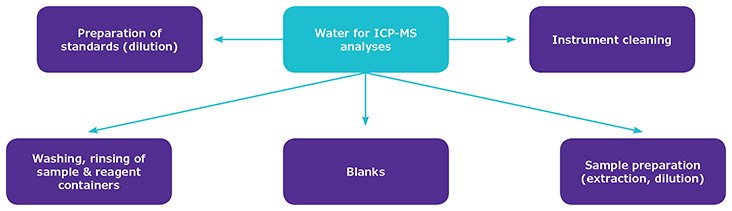
Figure 1.Various uses of ultrapure water in ICP-MS analysis
Trace Elemental Analysis of Milli-Q® Ultrapure Water
In the pharmaceutical industry, element analyses are performed in the range of mg/L (ppm) to sub-μg/L (sub-ppb) levels. As sensitivity, accuracy, precision and recovery must be appropriately demonstrated during the method validation process, achieving a low and stable background equivalent concentration (BEC) as well as low and consistent detection limits is critical. Table 1 presents the BEC and limit of detection (LOD) in Milli-Q® ultrapure water for each element in ng/L (ppt) level.
From Table 1, it can be observed that certain elements are present in slightly higher levels than sub-ppt. This can be explained by contamination coming from the laboratory environment, since the present analyses were performed under normal laboratory conditions.7 In case there is a need to achieve significantly lower levels of elements, it is reasonable to apply an additional polishing step, such as a Milli-Q® IQ Element purification unit which makes it possible to obtain BECs at sub-ppt and ppq levels in a clean room or metal-free laboratory environment.8
Elemental Impurity Testing: Experimental Conditions and Methods
Tap water was purified in two steps to obtain ultrapure water:
- Pure water was obtained from tap water thanks to the combination of intelligent reverse osmosis, Elix® electrodeionization (EDI), and a bactericidal UV lamp, using a Milli-Q® system similar to the Milli-Q® IX pure water system.
- Ultrapure water was obtained by further purifying the above water with a Milli-Q® polishing system, similar to the Milli-Q® IQ 7000 ultrapure water system, fitted with a Millipak® final filter. Note, for the analysis of mercury (Hg), ultrapure water was obtained from the Milli-Q® Direct system, which does not contain an Elix® EDI module.
The ultrapure water samples were analyzed as follows for levels of:
- V, Cr, Ni, Cu, As, Mo, Cd and Pb using an Agilent® 7700s ICP-MS instrument
- Hg, Ru, Rh, Os, Ir and Pt using an Agilent® 7500s ICP-MS instrument
All experiments were performed under regular laboratory conditions (not in a clean room).
Agilent® 7700s instrumental details and parameters: PFA-50 nebulizer, PFA spray chamber, sapphire inert torch, quartz 2.5 mm i.d. torch injector, platinum sample and skimmer cone, RF power 600/1600 W, sampling position 12 / 8 mm, carrier gas flow 0.90 L/min, makeup gas flow 0.32 / 0.51 L/min, auto detector mode, calibration through 1, 5, 10, 50 ng/L.
Agilent® 7500s instrumental details and parameters: quartz nebulizer, quartz spray chamber, quartz i.d. torch injector, nickel sample and skimmer cone, RF power 1300/1550 W, sampling position 8 mm, carrier gas flow 0.96 L/min, makeup gas flow 0.23 L/min, auto detector mode, calibration through 1, 20, 50, 100 ng/L.
Containers were all PFA pre-cleaned with ultrapure water. All ultrapure water samples (resistivity of 18.2 MΩ·cm and TOC below 5 ppb) from Milli-Q® water purification systems were analyzed immediately after water collection.
Conclusion: Suitability of Milli-Q® Ultrapure Water for Trace Analysis
Low levels of elemental impurities in ultrapure water produced by Milli-Q® ultrapure water purification systems were demonstrated. Laboratories in the pharmaceutical industry performing trace element analyses can rely on Milli-Q® water purification systems to produce high-purity water that meets their stringent requirements. Choosing water from Milli-Q® ultrapure water systems for element analyses will help to ensure the generation of accurate and reliable data.
Ultrapure Water Systems
Pure Water Systems
References
To continue reading please sign in or create an account.
Don't Have An Account?