Scalable, High-Quality Custom Oligonucleotides for Commercial PCR, qPCR & NGS
Custom oligonucleotides are foundational tools in PCR, qPCR, and genomic workflows where accuracy, consistency, and scalability matter. Oligos made under ISO guidelines are designed to support commercial and regulated applications by combining high-quality synthesis with robust quality systems and documentation. Whether you are transitioning from research to production or scaling an established assay, ISO-oligos provide the reliability, traceability, and performance needed to support confident manufacturing and long-term supply.
Section Overview.
- ISO-Oligo Considerations
- Securing Reliable, Scalable Custom Oligos for Commercial Use
- Quality Assurance for Custom Oligos
- Considerations for Your Commercial Custom Oligo Manufacturing Project
- Fundamental Commercial Capabilities for ISO Oligos
- Quality Control for Custom Commercial Oligos
- Custom Oligo Related Products
ISO-Oligo Considerations
What Are ISO-Oligos?
ISO-oligos are custom oligonucleotides manufactured under ISO-certified quality management systems, ensuring controlled synthesis, consistent performance, and comprehensive documentation suitable for commercial and regulated use.
How ISO-Oligos Differ from Standard Oligos
Unlike research-grade oligos, ISO-oligos are produced with:
- Tighter process controls and validated manufacturing workflows
- Enhanced lot-to-lot consistency and traceability
- Expanded quality documentation to support audits and regulatory submissions
- Scalable production capabilities for long-term supply
Recommended Use Cases
ISO-oligos are well suited for:
- PCR and qPCR assays used in regulated or commercial settings
- Diagnostic assay development and manufacturing
- Long-term assay supply where consistency is critical
- Transitioning assays from R&D into production
Design Considerations for Commercial Oligo Applications
When specifying custom ISO-oligos, consider:
- Sequence length, GC content, and melting temperature (Tm)
- Purification level required for assay performance
- Functional modifications or labels
- Required documentation and quality attributes for downstream compliance
Selecting ISO manufactured oligos helps ensure your oligonucleotides perform reliably not just today, but throughout the entire lifecycle of your assay.
Securing Reliable, Scalable Custom Oligos for Commercial Use
We offer custom primers, qPCR Probes, and Next-Gen Sequencing Oligos for Life Science Research Tools, Molecular Diagnostics (MDx), and Laboratory Developed Tests (LDTs). We also perform component or complete kit manufacturing, including custom formulations, packaging, and private labeling. Learn about our ISO and cGMP manufacturing capabilities or request a consultation to determine which type of product best fits your needs.
Quality Assurance for Custom Oligos
At the foundation of our ISO manufacturing processes is a robust Quality Management System (QMS), which drives compliance to our following Quality Registrations:
- ISO 9001:2015 for manufacturing research-grade oligonucleotides
- ISO 13485:2016 for manufacturing diagnostic-grade oligonucleotides
- ISO 14001:2015 for effective environmental management
This quality culture is integrated into all aspects of our business. Key components of our QMS include:
- Certificates of analysis
- Document control
- Change notification
- Vendor management
- Business agreements
- Corrective and preventive actions
Each component drives quality assurance, and for verification, we routinely host rigorous registrar, internal, and customer audits.
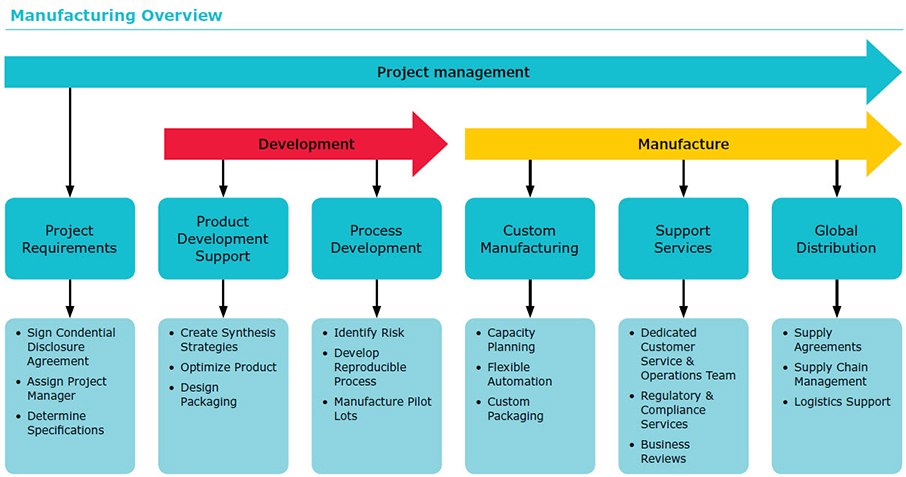
Oligo Project Management Partner
We believe that accountability, predictability, responsiveness, and flexibility are the keys to project success and customer satisfaction. A project management professional will work with you from the start of the custom oligo project through order fulfillment.
Process Development
Process development is a critical step in creating a robust and economical manufacturing process. To set process specifications, we:
- Identify critical control parameters and risks
- Develop consistent and reproducible processes
- Create specifications and manufacturing documents
- Provide small lots for stability testing, design verification, and design validation
- Manufacture the necessary pilot batches to achieve production scale
Our expertise in methods development ensures proven analytical procedures are integrated into our quality systems. Our multi-million dollar investment in state-of-the-art instrumentation and our experienced team of scientists are the foundation for our development and qualification process.
Reliable, Flexible Manufacturing
We have the personnel, infrastructure, equipment, and facilities to handle all of your manufacturing requirements. Our robust manufacturing processes are designed with our customers in mind. Our flexibility in manufacturing results from our technical expertise and core manufacturing capabilities, including:
- Flexible automation
- Range of production scales
- Stringent quality control
We understand that consistency is critical to manufacturing success. Our engineers design and maintain proprietary synthesis platforms, processing equipment and facilities. Instruments are qualified and an extensive preventive and predictive maintenance program ensures optimal performance. Utilizing our in-process controls and LIMS to capture information, process adjustments can be made in real time. Our manufacturing expertise makes us the partner of choice for designing and creating solutions for all your production needs.
Custom Formulations & Packaging
We can formulate the most complex components with precision and accuracy with the ability to:
- Formulate liquids and dry powders on a small to large scale
- Accommodate a variety of packaging configurations including microtiter plates, tubes and bottles
- Package a single component or an entire kit or assay to your specifications
- Private label your product
Custom Oligo Support Services
Our commitment to our partners is evident in all aspects of our business. We routinely tailor programs to meet custom oligo customer requirements including:
- Audits of any of our facilities
- Forecasting and capacity planning
- Quarterly business reviews
- Key performance indicators (KPIs)
- Inventory management services
- Global warehousing and distribution
Fundamental Commercial Capabilities for ISO Oligos
Whereas our research offering comes with breadth and speed, our commercial capabilities come with depth and consistency. Both product portfolios deliver high-quality oligonucleotides.
Custom DNA Oligos
Basic PCR primers, SYBR® Green Primers, isothermal amplification primers, sequencing primers, and more.
Custom qPCR Probes
Dual-Labeled Probes for the 5’ nuclease assay.
Quality Control (QC) for Custom ISO Oligos
Our ability to guarantee performance is directly related to our comprehensive understanding of oligonucleotide chemistry and synthesis platforms, our analytical systems, and our experience in methods development.
Quality control is integrated into the entire manufacturing process. Key checkpoints of quality control include:
- Starting materials & chemistry
- Instrumentation & synthesis
- Sequence identity verification by mass spectrometry
- Purify verification by analytical HPLC
- Quantification by UV spectroscopy
- Final inspection
To continue reading please sign in or create an account.
Don't Have An Account?