Aldol Condensation Reaction
What is Condensation Reaction?
The aldol condensation reaction is an organic reaction introduced by Charles Wurtz, who first prepared the β-hydroxy aldehyde from acetaldehdye in 1872.1 In an aldol condensation, an enolate ion reacts with a carbonyl compound in the presence of acid/base catalyst to form a β-hydroxy aldehyde or β-hydroxy ketone, followed by dehydration to give a conjugated enone. It is a useful carbon-carbon bond-forming reaction. The fundamental steps of the aldol condensation reaction are:
- Aldol (aldehyde + alcohol) reaction — Reaction of aldehyde (or ketone) enolate with another molecule of the aldehyde (or ketone) in the presence of NaOH or KOH to form β-hydroxy aldehyde (or ketone).
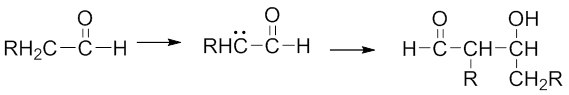
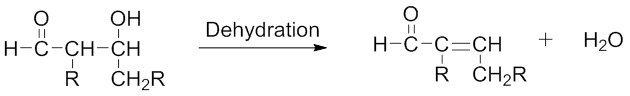
2. Dehydration/Elimination reaction — Involves removal of a water molecule from the β-hydroxy aldehyde (or ketone) to form an α,β-unsaturated aldehyde or an α,β-unsaturated ketone.
Precautions
Please consult the Safety Data Sheet for information regarding hazards and safe handling practices.
Applications
The Aldol condensation reaction can be used for the following syntheses:
- Enzymatic synthesis of fatty acids.2
- Highly concise total synthesis of epothilone B.3
- Preparation of (E)-6-(2,2,3-trimethyl-cyclopent-3-enyl)-hex-4-en-3-one.4
- Synthesis of high polymers of poly(glutaraldehyde).5
- Stereoselective synthesis of (±)-ephedrine.6
- Synthesis of numerous macrolide and ionophore antibiotics (natural products).7
- Total synthesis of distomadines A and B, two structurally unique tetracyclic quinolones.8
Recent Research and Trends
- Water-soluble calix[n]arenes were employed as inverse phase-transfer catalysts for aldol-type condensations and Michael addition reactions of activated methyl and methylene compounds.9
- A cesium ion containing catalyst, on an SBA-15 mesoporous molecular support, has been employed for the aldol condensation of methyl acetate with formaldehyde.10
- Organocatalytic asymmetric aldol reaction of cyclohexanone with p‑nitrobenzaldehyde in water has been reported.11
- One-pot Cu-catalyzed etherification/aldol condensation cascade reaction is reported to yield dibenzoxepine lactams.12
- Synthesis of a series of diprenylated and digeranylated chalcone analogues by alkylation, regioselective iodination, aldol condensation, Suzuki coupling and [1,3]‑sigmatropic rearrangement.13
- A domino sequence of Michael addition and aldol condensation of acenaphthenequinone with acetophenone in the presence of KOH in methanol solvent leads to the formation of different 2:2 adducts.14
- The aldol condensation of 4-isopropylbenzaldehyde and propanal has been performed using functionalized MCM-41.15
References
To continue reading please sign in or create an account.
Don't Have An Account?