Nicewicz Photoredox Catalysts for Anti-Markovnikov Alkene Hydrofunctionalization
Introduction
While Markovnikov alkene reactivity is very well developed and commonly utilized in the synthesis of commodity and research chemicals, catalytic access to the anti-Markovnikov-selective adducts remains a less-explored area in synthetic organic chemistry. In collaboration with Professor David Nicewicz, Sigma-Aldrich offers a selection of photo excitable acridinium salts that can facilitate various olefin hydrofunctionalizations with complete anti-Markovnikov selectivity.
The cationic organic oxidant 9-mesityl-10-methylacridinium perchlorate was originally designed by Shunichi Fukuzumi and co-workers.1 Since its introduction, the Nicewicz group has demonstrated the extensive utility of this material and related photoredox catalysts (794171, 793876, 793221) when employed alongside primarily thiol co-catalysts for hydrogen atom transfer. By generating alkene cation radical intermediates, this unique photoredox catalyst system enables hydrofunctionalization of a wide range of activated and unactivated alkenes with diverse nucleophiles, including carboxylic acids, amines, mineral acids, and propargylic and allylic alcohols.2–3
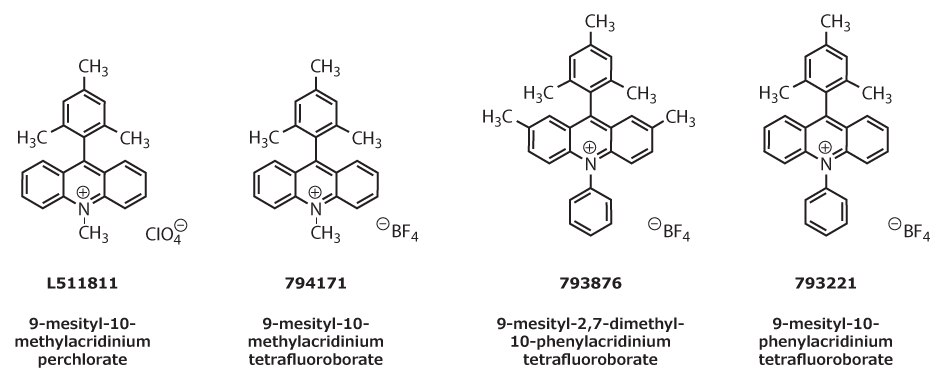
Figure 1.Structures of four acridinium salts with varied substituents and counterions used in propargylic and allylic alcohol reactions.
Advantages of Nicewicz Photoredox Catalysts
- Metal-free, visible light-mediated catalyst
- Stable to air and moisture
- Mild reaction conditions
- Anti-Markovnikov selectivity
- Superior oxidizing capabilities compared to Ru- and Ir-polypyridyl photooxidants
- Easy excitation of acridinium salts with LED flood lights
Hydroetherification
Complete regioselectivity was observed in over 10 examples of direct intramolecular anti-Markovnikov hydroetherification of alkenols when adding 9-mesityl-10-methylacridinium perchlorate with 2-phenylmalononitrile as a hydrogen atom donor.4
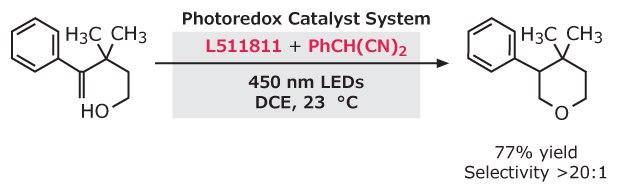
Figure 2.Hydroetherification of alkenols using 9-mesityl-10-methylacridinium perchlorate with 2-phenylmalononitrile under mild conditions, with a 77% yield and greater than 20.1 selectivity.
Hydroamination of Alkenes
Hydroamination of alkenes can be accessed using 9-mesityl-10-methylacridinium tetrafluoroborate (794171), with complete anti-Markovnikov selectivity in the presence of a thiol-containing co-catalyst. With thiophenol, intramolecular hydroamination of unsaturated amines provided access to several nitrogen-containing heterocycles.5 Furthermore, intermolecular hydroamination of aliphatic alkenes, α- and β-substituted styrenes, and heterocyclic amines were achieved with diphenyl disulfide.6
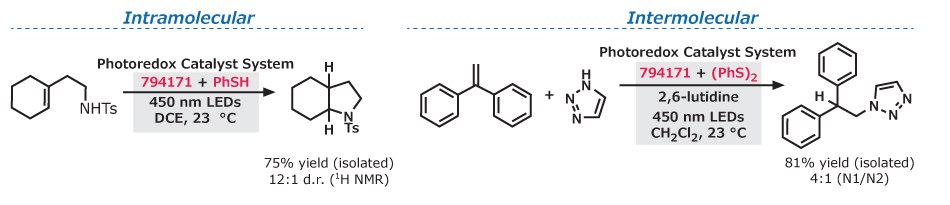
Figure 3.Intramolecular and intermolecular pathways are shown, highlighting the use of 9-mesityl-10-methylacridinium tetrafluoroborate along with a thiol containing co-catalyst to form nitrogen-containing heterocycles (left) and a 1,2,3-triazole functionalized disubstituted styrene derivative (right).
Hydrotrifluoromethylation
Diverse trifluoromethylated products have been obtained through this photoredox catalyst system. Single electron oxidation of the Langlois reagent (743232) by 9-mesityl-10-methylacridinium tetrafluoroborate (794171) and a thiol-containing co-catalyst (methyl thiosalicylate or thiophenol) results in the hydrotrifluoromethylation of styrenes and unactivated aliphatic alkenes with anti-Markovnikov selectivity. Nicewicz and co-workers provided 20 examples with 25–74% yields.7
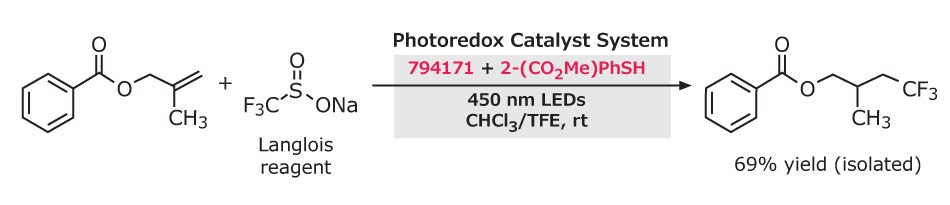
Figure 4.Hydrotrifluoromethylation of styrenes using Langlois reagent, 9-mesityl-10-methylacridinium tetrafluoroborate and methyl thiosalicylate under 450 nm LEDs in CHCl₃/TFE to give a CF₃–styrene product in 69% yield.
Hydroacetoxylation
Anti-Markovnikov-selective hydroacetoxylation of styrenes, enamides, and trisubstituted aliphatic amines is possible with a range of carboxylic acids when using 9-mesityl-10-methylacridinium tetrafluoroborate (794171) in combination with sodium benzene sulfinate or thiophenol. Seventeen reactions exhibited yields ranging from 29–99%.8
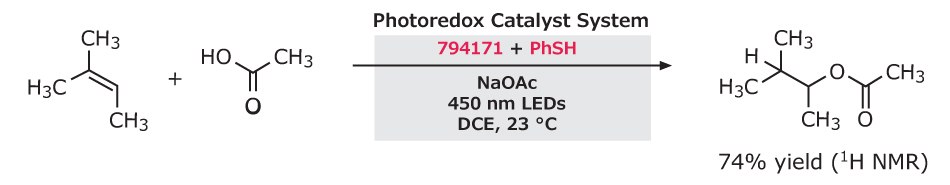
Figure 5.Anti-Markovnikov hydroacetoxylation of styrenes using 9-mesityl-10-methylacridinium tetrafluoroborate with PhSH, NaOAc, 450 nm LEDs in DCE at 23 °C, yielding 74% product.
Addition of Mineral Acids to Alkenes
The anti-Markovnikov addition of strong Brønsted acids to alkenes is achieved with complete regioselectivity using these photocatalysts. For hydrochlorination, 9-mesityl-10-methylacridinium tetrafluoroborate (794171) is used with thiophenol to generate the adduct. Hydrofluorination, hydrooxyphosphorylation and hydrooxysulfonylation are each effected by employing 9-mesityl-2,7-dimethyl-10-phenylacridinium tetrafluoroborate (793876) with the hydrogen-atom donors 4-nitrophenyl disulfide or 4-methoxythiophenol. Here, the inclusion of 2,6-lutidinium salts were found to provide the best reactivity through a nucleophilic couterion.9
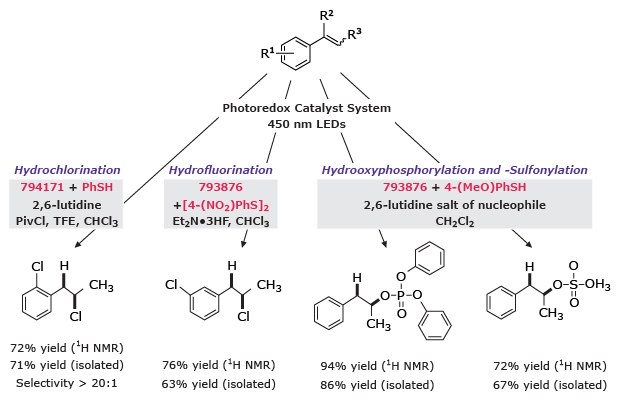
Figure 6.Photoredox functionalization of aromatics using 2,6-lutidinium salts: hydrochlorination (72%), hydrofluorination (76%), hydroxyphosphorylation (94%), and sulfonylation (72%) yields shown.
Intramolecular Hydrofunctionalization of Amides
The anti-Markovnikov intramolecular cyclization of unsaturated allylic amides and thioamides furnishes the resultant 2-oxazolines and 2-thiazolines, respectively. These transformations utilize a photoredox catalyst system comprising 9-mesityl-10-methylacridinium tetrafluoroborate (794171) in combination with diphenyl disulfide. Through 17 examples, a range of 59–82% yields were observed.10

Figure 7.Photoredox-catalyzed amide hydrofunctionalization using 9-mesityl-10-methylacridinium tetrafluoroborate with diphenyl disulfide in DCE under 450 nm LEDs for 14 h, yielding 76%.
Hydrodecarboxylation
The anti-Markovnikov hydrodecarboxylation of carboxylic and malonic acids is accomplished with 9-mesityl-10-phenylacridinium tetrafluoroborate (793221) and diphenyl disulfide. The decarboxylation reaction requires the inclusion of a base and a polar alcohol solvent, trifluoroethanol in order to afford good yields and scope. Eighteen examples were included to demonstrate the hydrodecarboxylation of primary, secondary, and tertiary carboxylic acids.11
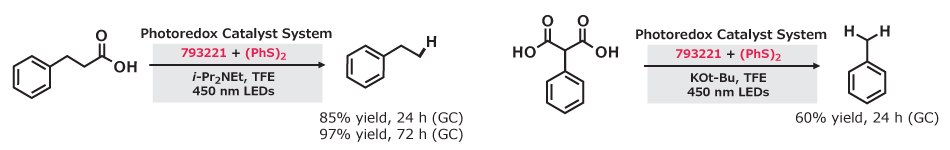
Figure 8.Photoredox hydrodecarboxylation of tertiary carboxylic acids (85% yield) and malonic acids (60–97% yield) using 9-mesityl-10-phenylacridinium tetrafluoroborate and (PhS)₂ under 450 nm LEDs.
Polar Radical Crossover Cycloadditions
In a tandem addition-cyclization sequence, polar radical crossover cycloadditions use variations of the photoredox catalyst system to generate the following products from alkenes: g-butyrolactones (with α,β-unsaturated carboxylic acids),12 tetrahydrofurans (with allylic alcohols),13 g-lactams (with unsaturated amides), and pyrrolidines (with unsaturated amines).14
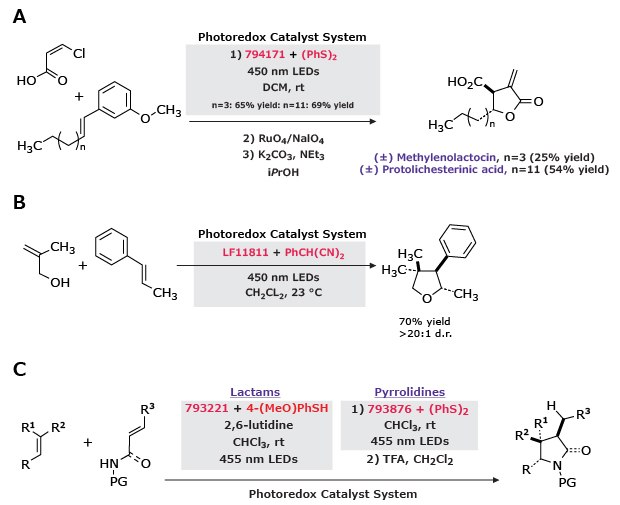
Figure 9.Polar radical crossover cycloadditions of alkenes using photoredox catalysis under visible light.
A) γ-Butyrolactones formed from α,β-unsaturated carboxylic acids under 450 nm LEDs, yielding methylenolactocin (25%) and protolichesterinic acid (54%).
B) Tetrahydrofurans generated from allylic alcohols in CH₂Cl₂ at 23 °C, affording 70% yield with >20:1 d.r.
C) γ-Lactams and pyrrolidines obtained from unsaturated amides and amines under 455 nm LEDs, giving 65–70% yields.
Photooxygenation
Prior to Nicewicz’s reports, Griesbeck and Cho used 9-mesityl-10-methylacridinium perchlorate in the presence of O2 as a visible-light mediated catalyst for Type II and electron-transfer photooxygenation reactions.15
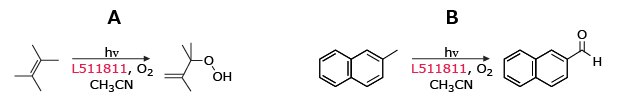
Figure 10.Visible-light-driven photooxygenation of A) alkenes via Type II (1O2) and B) aromatics via ET Type (O2•-) with L511811 in acetonitrile, yielding hydroxylated and carbonyl-containing products.
Related Products
References
To continue reading please sign in or create an account.
Don't Have An Account?