Water Contaminants: Types, Sources and Impacts in the Lab
Natural water is not simply the H2O molecule. It contains a variety of dissolved compounds originating from its contact with the atmosphere, rocks and soil, plants and living organisms. These impurities are typically classified as organic molecules, inorganic compounds, microorganisms or bacteria and their by-products, particles and colloids, and dissolved gas.
Tap drinking water is made from natural water and therefore contains these compounds, although government standards typically set maximum concentrations of harmful contaminants. Even if safe to drink, tap water may contain compounds that can impact laboratory experiments. For this reason, water must be further purified to be suitable for sensitive scientific analyses.
Origin of organic molecules in water
Dissolved organic molecules present in tap water are mainly of biological origin. For example, humic acids, tannins and lignan are by-products of the decay of plants. Man-made organic contaminants may also be present in tap water at trace levels. For instance, traces of pesticides, PFAS and pharmaceutical and personal care products are increasingly identified in potable water. Another example is the release of plasticizers by PVC pipes used to carry and deliver water to the tap.
Impact of organic ions in the laboratory
Water may be used to prepare solutions and reagents, to dilute standards, in many sample preparation methods, as blank, etc. Dissolved organics can affect techniques such as HPLC, UHPLC or LC-MS by causing baseline instability, decreased sensitivity and resolution, ghost peaks, and eventually reducing column lifetime. Cell culture or molecular biology experiments may also be affected by specific organic molecules, which may interfere with the hybridization process or act as endocrine disrupters.
Removal of organics from water
Several purification technologies can remove organic molecules from water, including reverse osmosis (RO), activated carbon, or photo-oxidation with ultra-violet (UV) irradiation.
Organic impurities in water are monitored by measuring the total oxidizable carbon (TOC, or total organic carbon). For trace organic analyses, water with a very low TOC (5 ppb or below) is recommended.

Inorganic ions commonly present in tap water are cations, such as sodium, calcium, magnesium and iron, and anions, such as bicarbonate, chloride and sulfate.
Origin of ions in water
Many of the minerals found in tap water come from nature and originate from the soil and rocks it has been in contact with. Depending on the water source, tap water’s mineral salt composition will vary. For example, in some areas water is considered hard, meaning that it contains high levels of dissolved minerals, primarily calcium and magnesium. Hard water can cause scale buildup in pipes and appliances, and leave spots on glassware.
Impact of inorganic ions in the laboratory
The presence of ions, even at trace levels, may affect elemental and inorganic analyses by causing inaccurate results or affecting experimental sensitivity. Techniques such as ion chromatography, atomic absorption spectroscopy (AAS) and ICP-MS require water that is free of ionic contamination. In addition, ions may affect biochemical or organic reactions by acting as catalysts or enzymatic cofactors (e.g. magnesium in PCR).
Removal of ions from water
Several purification technologies can remove ions from water, including reverse osmosis (RO), ion-exchange resins, and electro-deionization (EDI).
Ionic impurities in water are monitored by measuring its electrical conductivity or resistivity. A resistivity of 18.2 MΩ.cm indicates that the water is almost free from ions.
Bacteria are present in natural water, especially surface water. The pathogens responsible for waterborne diseases are usually eliminated from public water supplies by chlorination, ozonation or UV disinfection, but tap water still contains live micro-organisms.
Impact of bacteria and their by-products in the laboratory
Bacteria can cause different issues in laboratory experiments either directly or through their by-products, such as endotoxins, nucleases, proteases and alkaline phosphatase. They are known to interfere primarily with cell culture, molecular biology or immunodetection experiments, but almost any laboratory equipment will be affected by the proliferation of bacteria.
Removal from water
Several technologies can be used to control bacteria levels in purified water. First, filtration through a 0.22 µm screen filter or an ultrafilter can efficiently retain bacteria. In addition, irradiation with UV light can be used to inactivate bacteria. Bacterial by-products (endotoxins, nucleases, proteases or alkaline phosphatase) are much smaller in size than bacteria and can only be removed by ultrafiltration.

Origin of particles in water
Natural water usually contains soft particulates (such as plant debris) and hard particulates (sand, dust) as well as colloids (silica, colloidal iron or aluminum oxide, clay). They may also originate from minerals that precipitate when water is exposed to oxygen (e.g. calcium or magnesium carbonate).
Impact of particles and colloids in the laboratory
Suspended particles and colloids may cause turbidity, affecting any method relying on light beams (e.g. spectrophotometry). They may clog filters or deposit in instrument lines, columns or containers, causing the need for frequent cleanings or maintenance.
Particulates and colloids can interfere with instrument operation. For example, particulates can damage HPLC pumps and injectors, and increase system back pressure. They can also interfere with the measurement and analysis of micro- and nanoparticles, microplastics, etc.
Removal from water
For most applications, 0.22 µm filtration is adequate to remove particulates from purified water. In specific situations (when performing ICP-MS analyses or analyzing micro- or nanoparticles) additional filtration may be needed. In these situations, specific filters (0.1 µm or ultrafilters) may be recommended.

Most of the gases dissolved in natural water are absorbed from ambient air. Temperature and atmospheric pressure changes can impact gas dissolution. Water at sea level, being under higher pressure, contains more gases than water at higher altitudes.
Tap water contains dissolved gases such as nitrogen, oxygen and carbon dioxide. Oxygen, for instance, is absorbed from the atmosphere or may be produced by aquatic plants. Carbon dioxide may be generated by aquatic organisms or by microbial fermentation of organic matter. Other gases such as argon, nitrogen, methane or hydrogen sulfide may be present, but usually only at trace levels. Some gases may also be generated by human activity (agricultural or industrial).
Impact of dissolved gases in the laboratory
The concentration of oxygen in water can affect specific biochemical reactions. Dissolved gases can form bubbles that are detrimental to processes such as particulate counting or spectrophotometric measurements. They may also clog small tubing.
Removal from water
Benchtop water purification systems rarely include a degasser, and degassing, if required, is usually performed separately, just before water is used.
Even without a degasser, freshly purified ultrapure water with a resistivity of 18.2 MOhm.cm only contains very low levels of CO2. This is because CO2 is in equilibrium with carbonic acid, carbonate and bicarbonate in water (Figure 1), which are efficiently removed by deionization technologies. However, ultrapure water will quickly absorb CO2 from the air if it is left standing.
Contamination of purified water during storage
When stored, ultrapure water quickly absorbs any contamination present in the air of the lab.
In a classical experiment, ultrapure water with a resistivity of 18.2 MΩ.cm at 25 °C was left in a beaker and the evolution of its resistivity measured over time. The results are shown in Figure 1 and demonstrate the rapid contamination of water by carbon dioxide from the air, leading to the production of HCO3- in the water and the resulting decrease of resistivity from 18.2 MΩ.cm to 4 MΩ.cm in about one hour.
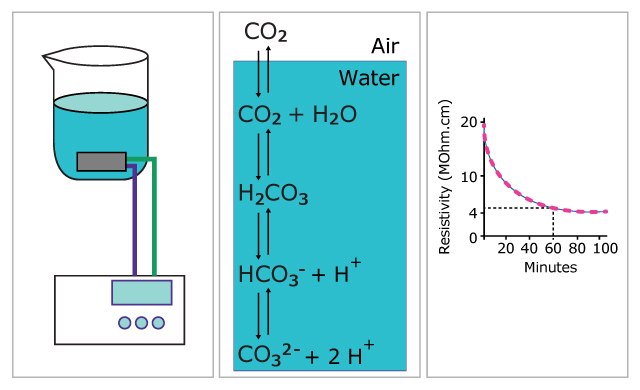
Figure 1. Demonstration of the contamination of ultrapure water with carbon dioxide from the air.
What is true for carbon dioxide is also true for other chemical substances that may be present in the air of a laboratory, such as acid fumes (from nitric acid or chlorohydric acid) and volatile solvents (such as toluene, acetone or tetrahydrofuran). Purified water is also easily contaminated by materials extracted from containers, such as sodium and silica from glass, plasticizers and ions from polymeric materials (for instance, phthalate esters from PVC pipes, fluoride from PTFE pipes) and metallic ions from metallic containers.
For this reason, to minimize risks of contamination and experimental variability that can be caused by these contaminants, ultrapure water should be produced just before use and used as soon as possible.
Contact us if you would like support from a lab water expert in selecting a water system for your lab.
Related Products
To continue reading please sign in or create an account.
Don't Have An Account?