What is Click Chemistry? An Introduction
Accelerating Drug Discovery with Click Chemistry
The traditional drug discovery process, reliant on natural secondary metabolites, has often been slow, costly, and labor-intensive. Despite advancements in combinatorial chemistry and high-throughput screening, generating lead compounds still depends on the reliability of individual reactions to construct new molecular frameworks.
Click Chemistry Reaction Mechanisms
Click chemistry is a modern approach to the synthesis of drug-like molecules that accelerates the drug discovery process by utilizing a few practical and reliable reactions. Sharpless and coworkers defined a click reaction as one that is broad in scope, easy to perform, uses readily available reagents, and is insensitive to oxygen and water. In many cases, water serves as the ideal reaction solvent, providing optimal yields and rates. The work-up and purification process employs benign solvents and circumvents the need for chromatography, making the process more efficient and sustainable.1
Click Chemistry Reaction Processes
- Simple to perform
- Modular
- Broad scope of applications
- High yielding reactions
- Stereospecific
- Adheres to the 12 Principles of Green Chemistry by generating harmless byproducts, removable by nonchromatographic methods
Advantages of Click Chemistry1
- Simple reaction conditions
- Readily and easily available starting materials and reagents
- Use of no solvent, a benign solvent (such as water), or one that is easily removed
- Simple product isolation
- Product should be stable under physiological conditions
Click chemistry employs a modular approach and has significant applications in drug discovery, combinatorial chemistry, target-templated in situ chemistry, and DNA research.1
Examples of Azide-Alkyne Click Chemistry Reactions
Azide click chemistry is exemplified by the Cu(I)-catalyzed Huisgen 1,3-dipolar cycloaddition of terminal alkynes with organoazides to yield 1,4-disubstituted 1,2,3-triazoles (Scheme 1).2 This reaction is known for its reliability, high yield, ease of execution, and tolerance to air, moisture, and various functional groups. Water often serves as the ideal solvent, offering the best yields and highest reaction rates. Typically, the resulting cycloadducts are solids, eliminating the need for chromatographic purification. The 1,2,3-triazole ring is resistant to hydrolysis, oxidation, reduction, and other cleavage modes, making the Cu(I)-catalyzed azide-alkyne cyclization a crucial tool in drug discovery library development.1,3-4
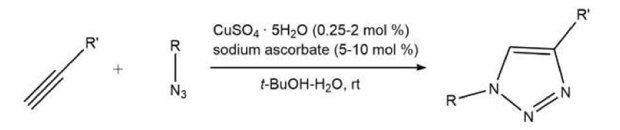
Scheme 1.Copper-catalyzed azide-alkyne cycloaddition reaction (CuAAC)
For the preparative synthesis of 1,2,3-triazoles, using a Cu(II) salt with an ascorbate reducing agent to form catalytically-active Cu(I) is preferred. However, this method can pose challenges in bioconjugation applications. Alternatively, a Cu(I) salt like [Cu(CH3CN)4]PF6 can be used directly with the stabilizing ligand tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine, TBTA (Scheme 2).8 TBTA effectively enhances the copper-catalyzed cycloaddition without damaging biological scaffolds. 5-7
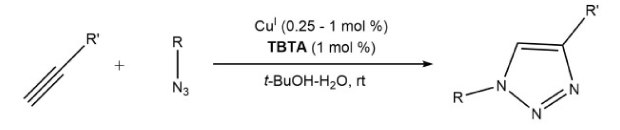
Scheme 2.Copper(I)-catalyzed synthesis of 1,2,3-triazoles
While the Cu(I)-catalyzed reaction yields 1,4-disubstituted triazoles, a transition metal variant can produce the complementary 1,5-isomer. Treating a terminal or internal alkyne with an azide in the presence of catalytic Cp*RuCl(PPh3)2 results in a high-yield cycloadduct with complete regiospecificity control (Scheme 3).4,9
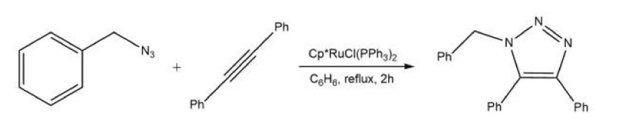
Scheme 3.Ruthenium-catalyzed one step azide-alkyne cycloaddition (RuAAC)
Examples of Copper-Free Click Chemistry Reactions
A well-known example of a copper-free click chemistry reaction is the strain-promoted azide-alkyne cycloaddition (SPAAC). This reaction occurs between azides and strained cyclooctynes, such as difluorinated cyclooctyne (DIFO), without requiring a copper catalyst. The mechanism is driven by the significant ring strain in the cyclooctyne, which creates a highly reactive intermediate. When the azide reacts with this strained alkyne, the cycloaddition proceeds via a concerted mechanism, where the azide undergoes nucleophilic attack on the carbon-carbon triple bond, leading to the formation of a stable 1,2,3-triazole product. The strain in the cyclooctyne ring accelerates the reaction under physiological conditions, making it ideal for bioconjugation in living systems while avoiding potential toxicity from copper ions.
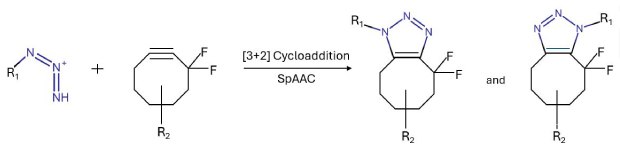
Scheme 4.Strain-promoted azide-alkyne cycloaddition (SPAAC)
Related Products
References
Para seguir leyendo, inicie sesión o cree una cuenta.
¿No tiene una cuenta?