Integrating Multiplex and High Sensitivity Immunoassay Detection of Alzheimer’s Disease Biomarkers
Understanding complex neurodegenerative diseases, like Alzheimer’s Disease (AD), requires powerful tools for biomarker detection. By integrating multiplex and high-sensitivity immunoassays, researchers can gain deeper insights into the underlying mechanisms of disease. Explore how this approach was put into action using MILLIPLEX® multiplex and SMC® high-sensitivity immunoassays to detect Alzheimer’s biomarkers in human cerebrospinal fluid, plasma, and serum samples.
Why Should You Combine Multiplex and High Sensitivity Immunoassays in AD Research?
Monitoring protein biomarkers in cerebrospinal fluid (CSF) of individuals with AD has been highly beneficial in understanding disease progression1. Multiplex immunoassays allow researchers to measure several biomarkers simultaneously and generate an abundance of reliable data from a single experiment. But, while detection of several CSF biomarkers can reproducibly distinguish between apparently healthy and AD samples, obtaining CSF poses a challenge in some research studies.
The need for more accessible blood-based AD biomarkers has driven a continuous search for novel candidates2. However, the sensitivity of standard immunoassay methodologies may limit the identification of protein biomarkers in serum and plasma. High-sensitivity immunoassay technologies can transform neurodegenerative disease research by enabling the measurement of low-abundant proteins in a variety of biofluids and by creating opportunities for the identification of novel biomarkers3,4.
How to Use Multiplexing Immunoassays with High Sensitivity Immunoassays
To demonstrate the value of pairing the ultrasensitive capabilities of SMC® technology with Luminex® xMAP® multiplexing, we quantified AD biomarkers in diverse sample types. Biofluids from apparently healthy controls and individuals with AD were evaluated using commercially available MILLIPLEX® and SMC® neuroscience kits.
The human CSF, plasma, and serum samples were obtained from commercial vendors (Discovery Life Sciences, BioIVT, and PrecisionMed) and tested neat or diluted, according to the kit protocol. An unpaired t-test was used to calculate two-tail p-values for all analytes (GraphPad Prism).
MILLIPLEX® multiplex assays were performed in 96-well plates according to the product instruction manuals. Mean Fluorescence Intensity (MFI) was measured on a Luminex® 200™ system and data were analyzed with MILLIPLEX® Analyst 5.1 Software.
The following multiplex assays were used:
- MILLIPLEX® Human Amyloid Beta and Tau Panel (Prod. No. HNABTMAG-68K) was used to quantitate the analytes: Amyloid β (1-40) (Aβ40), Amyloid β (1-42) (Aβ42), total Tau (tTau), and pTau T181 in 1:2 diluted CSF samples.
- MILLIPLEX® Human Neuroscience Panel 1 (Prod. No. HNS1MAG-95K) was used to quantitate the analytes: α-Synuclein, Glial Fibrillary Acidic Protein (GFAP), Neuron-Specific Enolase (NSE), PARK5 (UCHL1), PARK7 (DJ-1), and Transglutaminase 2 (TGM2) in neat CSF samples.
- MILLIPLEX® Human Neuroscience Panel 2 (Prod. No. HNS2MAG-95K) was used to quantitate the analytes: Angiogenin (ANG), ApoE4, FABP3, Ferritin, Neurogranin (NGRN), and TREM2, in 1:10 diluted CSF, serum, and plasma samples. This panel has been verified for both CSF and blood (serum and plasma) samples.
SMC® Amyloid Beta 1-40 High Sensitivity Immunoassay Kit (Prod. No. 03-0145-00) and SMC® Amyloid Beta 1-42 High Sensitivity Immunoassay Kit (Prod. No. 03-0146-00) were used to quantitate amyloid beta peptides according to product instruction manuals using the SMCxPRO® instrument.
Multiplexing Results
MILLIPLEX® multiplex immunoassays are recognized as valuable tools to measure biomarkers of neurodegenerative disease. Simultaneous detection of multiple proteins not only conserves sample, it also provides a more complete picture of disease pathways.
CSF Samples
Several established and emerging neurodegenerative disease biomarkers were examined in the CSF samples of apparently healthy controls and individuals with AD, before assessing biomarker detection in serum and plasma samples. Standard curves for each of the kits that were used are shown in Figure 1.
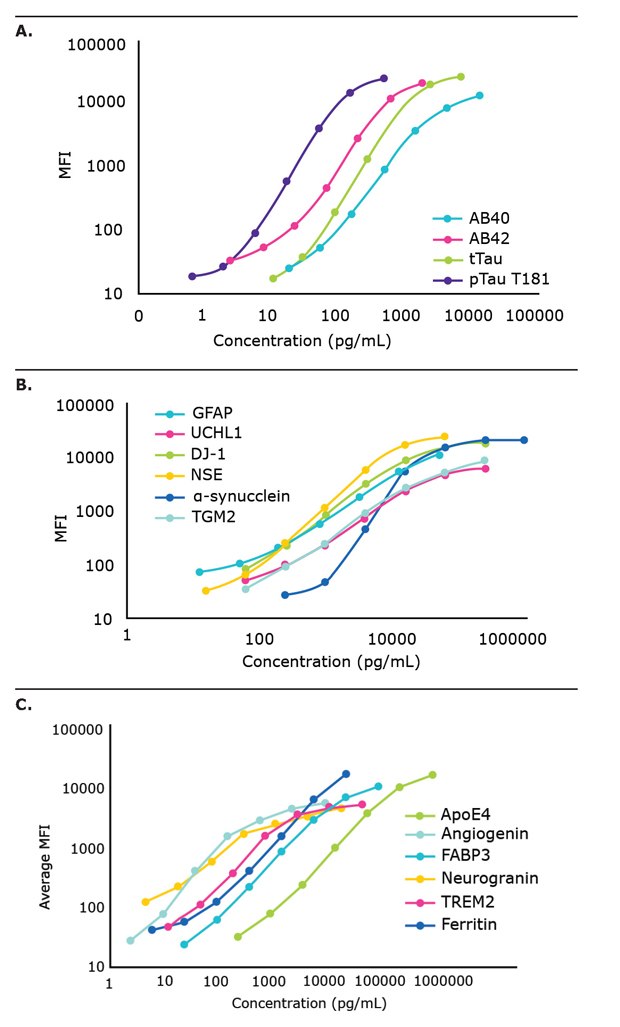
Figure 1.Multiplex immunoassays for neuroscience research. Standard curves for the MILLIPLEX® (A) Human Amyloid Beta and Tau Kit, (B) Human Neuroscience Panel 1, and (C) Human Neuroscience Panel 2 are shown.
Sample analysis, detected with multiplex panels, identified distinct differences in biomarker concentrations. Each kit highlighted notable changes in AD samples relative to controls (Figure 2).
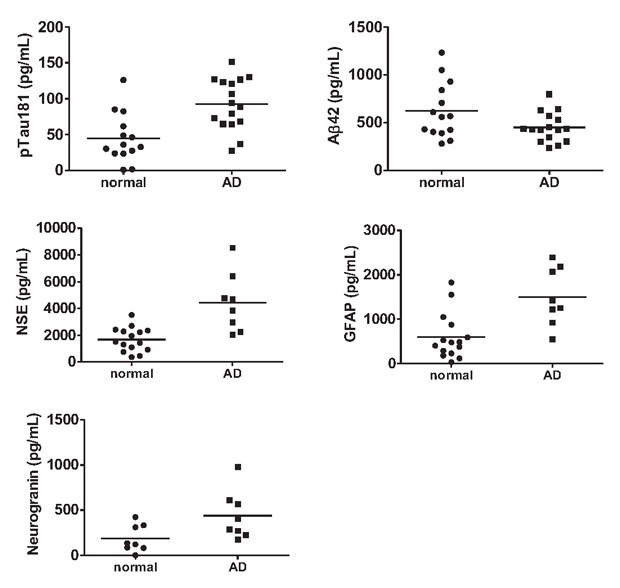
Figure 2. Multiplex biomarker analysis of apparently healthy control and AD CSF samples.
MILLIPLEX® neuroscience kits were used to measure a total of 16 proteins in human CSF. Analysis of apparently healthy control and AD CSF samples with each of these kits revealed differences in phosphorylated Tau (p=0.0009), Aβ42 (p=0.0476), NSE (p=0.008), GFAP (p=0.0016), UCHL1 (p=0.0088, data not shown), and Neurogranin (p=0.0359).
For each kit, sample sizes are as follows:
- Human Amyloid Beta and Tau Panel - control CSF (n=14) and AD CSF (n=16)
- Human Neuroscience Panel 1 – control CSF (n=15) and AD CSF (n=8)
- Human Neuroscience Panel 2 - control CSF (n=7) and AD CSF (n=7)
In the Human Amyloid Beta and Tau Panel, Aβ42 was decreased in AD CSF, whereas phospho-Tau (Thr181) was increased, consistent with well-established observations of these canonical AD biomarkers5. NSE and GFAP from Human Neuroscience Panel 1 were both increased in AD CSF. In Human Neuroscience Panel 2, neurogranin NRGN exhibited higher levels in AD CSF relative to apparently healthy controls.
Serum and Plasma Samples
As Human Neuroscience Panel 2 is also verified for serum and plasma samples, we next examined whether any of the analytes exhibited differential protein expression in circulation. This assessment of AD plasma and serum (n=5 and 6, respectively) versus apparently healthy control plasma and serum (n=12 and 8, respectively) pinpointed increases in TREM2 (serum p=0.008), FABP3 (serum p=0.020, plasma p=0.002), and neurogranin (plasma p=0.004) in AD samples relative to apparently healthy controls (Figure 3).
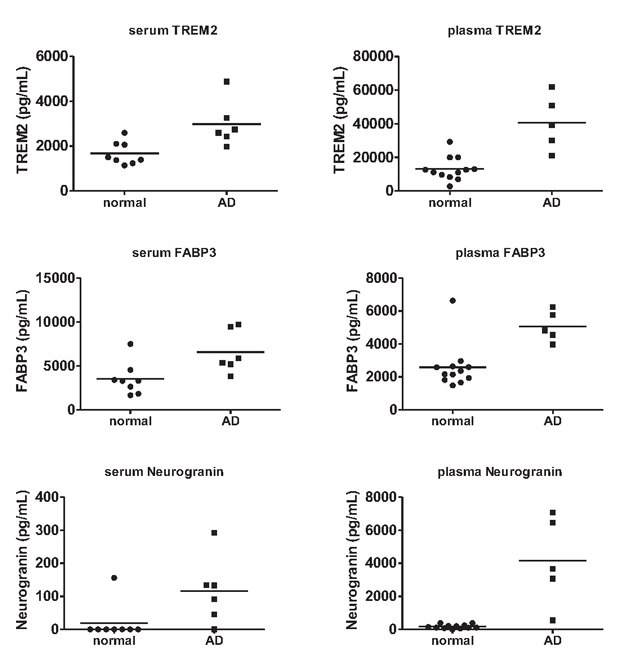
Figure 3. Multiplex AD biomarker measurement in apparently healthy control and AD plasma and serum samples.
High Sensitivity Results
While several analytes demonstrated potential as blood-based biomarkers of AD, the low abundance of other proteins necessitated the development of assays on a high-sensitivity platform using SMC® technology. SMC® technology enabled the measurement of the amyloid beta peptides in human CSF and plasma samples from apparently healthy donors and individuals with AD (Figure 4). Measured with their respective high-sensitivity SMC® immunoassays, a small increase in Aβ40 was seen in AD plasma values versus control samples with no difference observed between serum Aβ42 levels in AD and control samples. Importantly, the SMC® Aβ42 kit effectively identified the decrease in CSF Aβ42 levels (p=0.0116), a hallmark feature of AD.
For each kit, sample sizes are as follows:
- SMC® Amyloid Beta 1-40 High Sensitivity Immunoassay Kit apparently healthy control CSF (n=20), AD CSF (n=10), apparently healthy control plasma (n=10), AD plasma (n=10)
- SMC® Amyloid Beta 1-42 High Sensitivity Immunoassay Kit apparently healthy control CSF (n=8) and AD CSF (n=8), apparently healthy control plasma (n=10), and AD plasma (n=10)

Figure 4. High sensitivity immunoassay biomarker measurement in apparently healthy control and AD plasma and CSF samples.
Summary
MILLIPLEX® and SMC® immunoassays were used to measure a multitude of proteins linked to neurodegenerative disease in CSF, plasma, and serum samples. Established AD biomarkers such as Aβ42 and phospho-Tau showed the expected patterns of alteration in CSF. Additionally, proteins such as Neurogranin and GFAP demonstrated elevated concentrations in AD CSF. Potential blood-based biomarkers of AD were analyzed in plasma and serum samples, with several promising candidates emerging. Future studies will be integral to determining whether these observations can be corroborated with larger sample cohorts.
By adopting a fit-for-purpose approach, multiple technology platforms and immunoassays were integrated to analyze AD biomarkers in CSF, plasma, and serum samples. These MILLIPLEX® and SMC® immunoassays provide researchers with reliable resources for studying a breadth of neurodegenerative disease biomarkers in a variety of biofluids.
Related Products
References
To continue reading please sign in or create an account.
Don't Have An Account?