Purification of Extracellular Vesicles with Eshmuno® Q Resin and Natrix® Q Membrane
Introduction
Extracellular vesicles (EVs), small, membrane-bound particles released by cells into their surrounding environment, are key mediators of intercellular communication. They carry a variety of biomolecules, including proteins, lipids, and genetic material, and play critical roles in physiological processes like immune regulation, tissue repair, and development, as well as in pathological conditions such as cancer progression, inflammation, and neurodegenerative diseases.1 The ability of EVs to transfer biomolecules between cells has attracted interest in their use as novel therapeutic vehicles.
EVs exist in diverse subpopulations, defined by unique characteristics such as size, density, lipid composition, membrane proteins, and cargo.2 These variations pose significant challenges to purification and have hindered progress in developing EVs as a therapeutic modality, primarily due to the absence of a standardized, scalable isolation method.
This proof-of-concept study demonstrates the successful isolation of EVs derived from HEK-293T cells using anion exchange technologies with either Eshmuno® Q resin or a Natrix® Q chromatography membrane.
Section Overview
- Eshmuno® Q Anion Exchange Resin
- Natrix® Q Chromatography Membrane
- Experimental Results
- Separation and Recovery of EVs Utilizing Eshmuno® Q AEX Resin
- Size Distribution and Protein Contamination during Elution
- Reduction of Co-eluting Contamination through Benzonase® Endonuclease Treatment
- EV Purification Using Natrix® Q Chromatography Membrane
- Conclusion
- Related Products
Eshmuno® Q Anion Exchange Resin
Eshmuno® Q is a strong anion exchange (AEX) resin featuring trimethylammoniumethyl ligands on a surface-grafted, rigid, hydrophilic polyvinyl ether polymer base matrix. This robust structure provides exceptional pressure stability, enabling high flow rates that accelerate processing of large volumes of fluids, reducing process times and production costs. Utilizing tentacle technology, Eshmuno® resins enhance functional group accessibility by minimizing steric hindrance. Their linear polymer chains optimize interactions with target molecules, improving the isolation of large biomolecules such as antibodies, viruses, and EVs. This design supports higher binding capacities, ensuring more efficient purification.
Learn more about Eshmuno® Q Anion Exchange Resin.
Natrix® Q Chromatography Membrane
Natrix® Q chromatography membrane consist of porous polyacrylamide hydrogel containing a high density of pendant quaternary ammonium (Q) ligands physically reinforced by an inert fiber web.
The interconnected pore structure and high ligand density provide a large surface area for protein binding, contributing to high permeability and superior productivity and throughput. This design enables very fast flow rates and residence times of 6 seconds allowing for high loads while maintaining excellent impurity clearance under fast operating conditions. In combination with the well-established Q ligand chemistry, these features make Natrix® chromatography membrane an excellent choice for reliable large-scale purification of EVs.
Learn more about Natrix® Q Chromatography Membrane.
Experimental Results
Chromatographic Profile of EVs
HEK293-derived EVs can bind to and be eluted from an Eshmuno® Q resin (Figure 1). Loading of concentrated and Benzonase® endonuclease-treated conditioned media and linear elution starting at 125 mM up to 1.2M of NaCl revealed different chromatographic profiles between multi-angle light scattering (MALS) and UV detection. While UV adsorption at 260nm showed no variations between runs during loading, the MALS signal varied, indicating different amounts of particles present in the FT. UV absorption during salt gradient revealed one peak, whereas the MALS detector revealed two partially resolved peaks. Both signals showed a narrow peak at the beginning of the cleaning in place (CIP). All fractions were analyzed using various techniques for in-depth characterization of all chromatographic steps.
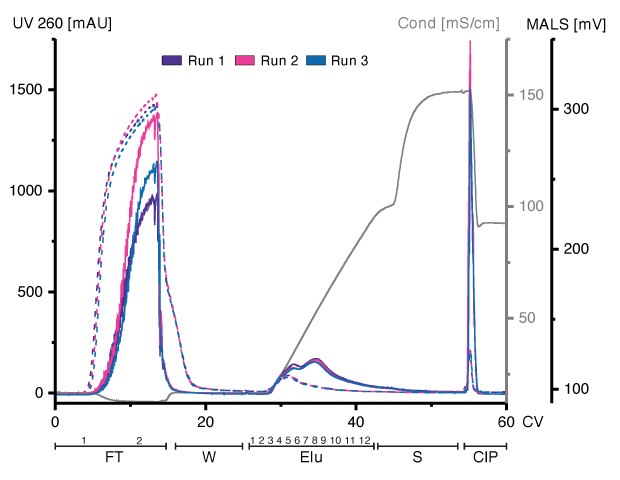
Figure 1.Overlay of three chromatograms of individual replicates, run 1 (purple), run 2 (pink), and run 3 (blue) in column volumes (CV) with chromatographic steps flow-through (FT), wash (W), elution (Elu), strip (S) and cleaning-in-place (CIP)and including fraction numbers. UV 260: dashed line; MALS traces: continuous line), conductivity: light grey.
Separation and Recovery of EVs utilizing Eshmuno® Q AEX Resin
CM as load and subsequent fractions were analyzed for particle concentration and size distribution using nanoparticle tracking analysis (NTA). Particles were detectable in FT, Elu, S, and CIP fractions, with a total recovery of 70-80% (Figures 2A and 2B).
Recovery and elution profiles of EVs were also monitored using ELISA, targeting the common EV marker CD81 on intact EVs. Overall recovery based on CD81 quantification (Figures 2C and 2D) was slightly lower than NTA quantification. Elution profiles followed similar profiles with sharper drop-off towards the end of the elution. In S and CIP fractions, CD81 was almost absent.
Size Distribution and Protein Contamination during Elution
Fractions were analyzed for protein content by BCA to investigate the co-elution of proteins (Figure 2E). Unpurified EVs had the highest protein concentration, followed by the FT. Protein concentration in the elution fraction increased until Elu 4 and then decreased again. High-salt concentrations during the S step did not elute proteins, while the CIP step removed residual proteins from the column. Particle size, determined by NTA, increased during FT in comparison to Load, while elution displayed smaller particles with an increase in size throughout the elution. High-salt during S eluted bigger particles while CIP eluted on average smaller particles with a higher size range.
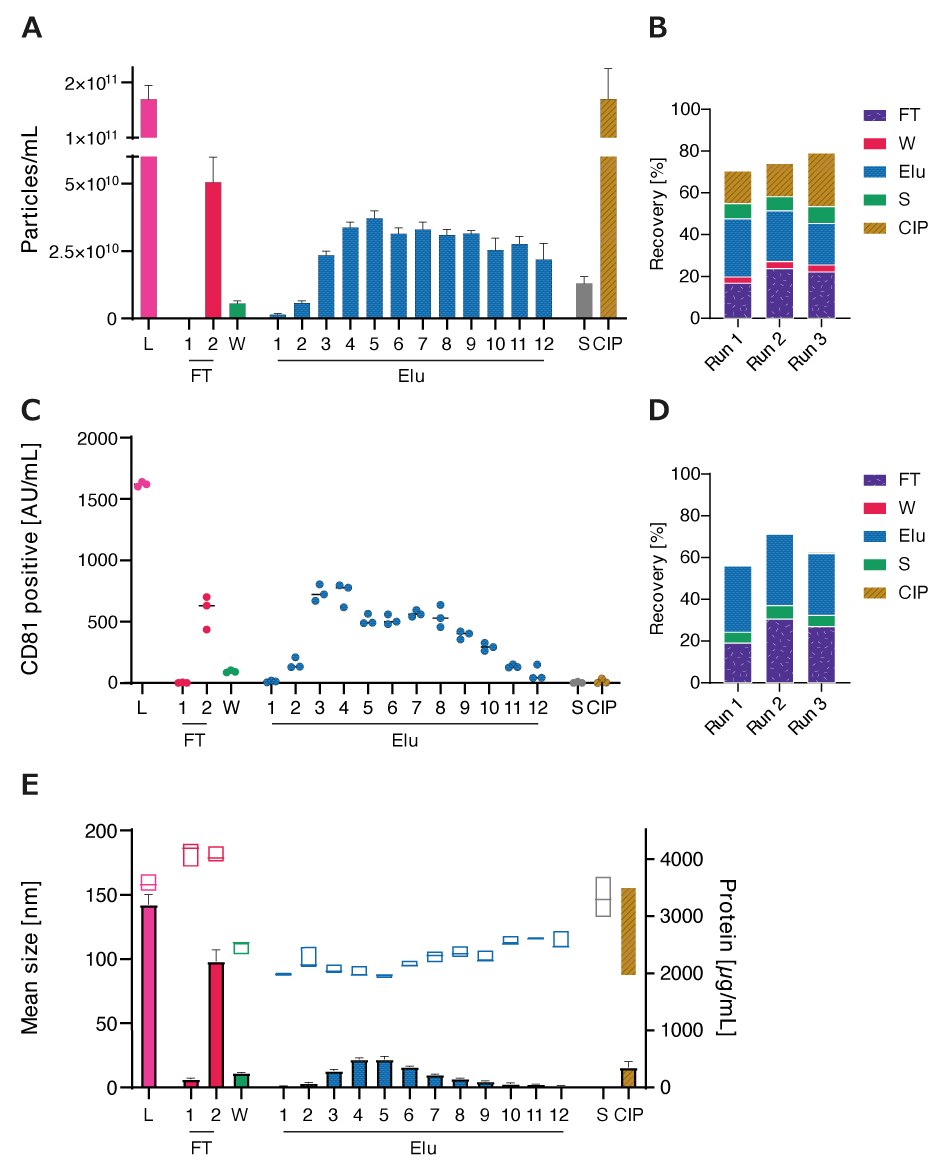
Figure 2.A) Particle concentrations measured by nanoparticle tracking analysis (NTA) of load (L) and all chromatographic fractions flow-through (FT), wash (W), elution (Elu), strip (S) and cleaning in place (CIP) B) Recovery of chromatographic steps based on NTA data. Data presented are the mean +/- SEM of three single replicates with five technical replicates. C) Analysis of chromatographic fractions based on cluster of differentiation (CD)81 presence by CD81 enzyme-linked immunosorbent assay (ELISA). D) Recovery of chromatographic steps based on ELISA data. Data presented are the mean +/- SEM of three single replicates with two technical replicates. Protein concentration, measured by bicinchoninic acid assay (BCA), and particle size, measured by NTA, of load (L) and chromatographic fractions flow-through (FT), wash (W), elution (Elu), strip (S) and cleaning in place (CIP). E) Purity of isolated HEK293 EV (bars) and mean particle size (boxes).
Reduction of Co-eluting Contamination through Benzonase® Endonuclease Treatment
Chromatin, a DNA and protein complex, exhibits an overall negative charge, which can interact with positively charged functional groups found on Eshmuno® Q resin. Use of Benzonase® endonuclease treatment drastically reduced the amount of DNA and binding of associated proteins to Eshmuno® Q resin during loading which then reduced the levels of impurities co-eluting with EVs (data not shown).
EV Purification Using Natrix® Q Chromatography Membrane
Figure 3 shows the separation of EVs using a Natrix® Q AEX membrane and resulting particle concentrations of all fractions. Approximately 40-50% of particles were recovered during elution in two nearly separated peaks.
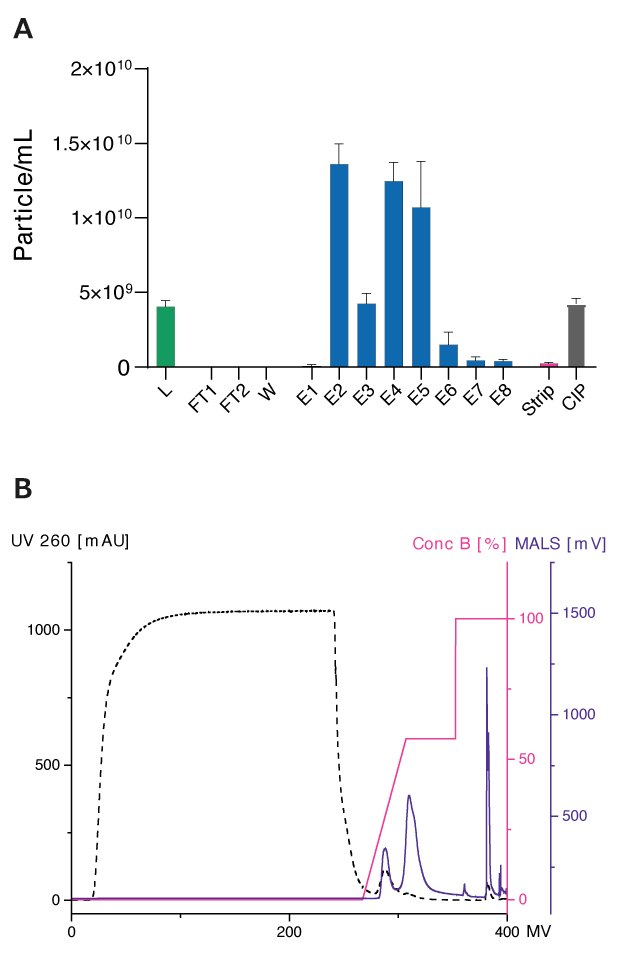
Figure 3.Benzonase-treated and filtrated conditioned HEK293 medium were loaded (L) onto the membrane with 200 μL membrane volume. The flow rate of 1 mL/min were used. Elution was performed using a Tris buffer at pH 7.4 and a linear salt gradient with NaCl (150 – 1200 mM). For the Strip step, a Tris buffer at pH 7.4 with 2 M NaCl was used and CIP was performed using 0.5 M NaOH. Particle concentrations were analyzed using Flow NanoAnalyzer with NF Profession 2.0. Laser power was set to 6 mW at 488 nm. Sampling pressure was set to 1 kPa with a recording time of 60 sec. Data shown represents the mean +/- SEM of three individual replicates with two technical replicates.
Conclusion
Heterogeneous bulk isolates of EVs are commonly used in pre-clinical and clinical studies, which limits their therapeutic potential. Compounding these challenges, current methods for separating EV subpopulations are either not scalable or fail to achieve adequate purity and yield. Developing scalable, high-purity purification processes is essential to unlock the full potential of EVs as carriers of therapeutic biomolecules, paving the way for transformative advancements in medicine.
IEX chromatography is a well-established method in the biopharmaceutical industry for downstream purification processes and can be used to separate EV subpopulations based on specific surface charges and their interaction with the functional groups on the resin. This study describes the use of Eshmuno® Q ion exchange resin for the direct isolation and purification of EVs from cell culture supernatants. The isolated EVs showed a reduction in protein contamination during the elution process as compared to the feed. EVs were separated into two peaks, with tetraspanin-positive EVs eluting in the first peak, containing more protein and a smaller average particle size. The second peak contained fewer tetraspanin-positive EVs and less total protein but had larger particle sizes. However, the tetraspanin composition remained constant throughout the elution. Additionally, use of Benzonase® endonuclease treatment before chromatographic purification significantly improved the purity of isolated EVs and affected their elution profile.
Natrix® Q membrane technology offers the further advantage of high flow rates, enabling EV purification of cell culture supernatants to be performed with short process times without prior concentration. This scalable approach improves the ability to purify EVs, advancing the potential to leverage them as carriers of therapeutic biomolecules.
Discover our IEX chromatography resins and our membrane chromatography devices.
Note:
For complete information on the materials and methods related to the production and isolation of EVs, please refer to Koch, et al.2
Related Products
References
To continue reading please sign in or create an account.
Don't Have An Account?