Milli-Q® Clinical Water Purification Systems
Reliable water purification is critical for clinical laboratories to comply with CLSI® standards and for smooth and uninterrupted daily lab operations.
Here’s how the range of Milli-Q® clinical water purification systems deliver robust performance to support laboratory productivity, efficiency and the reliability of patient results:
- Assure consistent-quality Clinical Laboratory Reagent Water (CLRW)
- Decrease operating expenses thanks to reduced water use
- Reduce downtime by eliminating maintenance procedures (e.g. no resin cylinder changes)
- Save time thanks to simplified use, data traceability and maintenance
- Achieve more uptime with real-time online monitoring and best-in-class Milli-Q® Services
Products
Choose from a Range of Adaptable Water Solutions
In every clinical lab, whether in a large testing facility, hospital or small clinic, the water system set up must:
- Comply with quality requirements of the analyzers and their various applications
- Meet volume demands
- Fit the laboratory’s space
We offer a range of water purification systems that can be adapted to any lab’s specifications. No matter the lab size, set up, or quality requirements, solutions are available that produce from a few liters to up to several thousand liters per day of pure, CLRW, and ultrapure quality water.
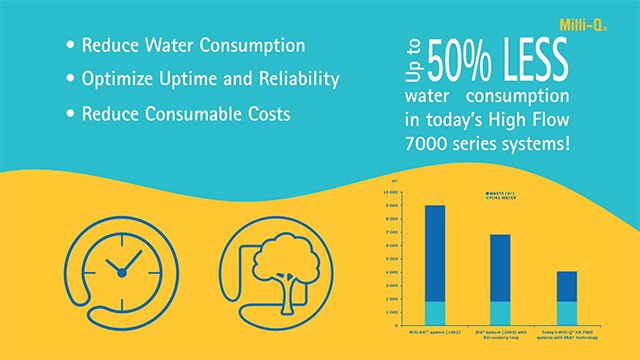
Reduce Running Costs & Environmental Impact
Innovative Milli-Q® systems are designed to reduce water, chemical waste, and associated costs:
Up to 50% water savings
Recycling of reject reverse osmosis (RO) water by E.R.A.® (evolutive reject adjustment) technology reduces water use up to 50% versus traditional RO systems. This process also extends the lifetime of pre-treatment cartridges, further reducing laboratory waste.
No chemical waste
Elix® electrodeionization (EDI) technology eliminates the need for hazardous chemical regeneration procedures, associated waste, and frequent replacement of costly resin cylinders.

Quick and easy cartridge changes thanks to the patented, ergonomic pack-locking system on Milli-Q® high-flow water systems.
Achieve More Efficient Daily Operations
Less time spent managing lab equipment, including water systems, can save precious time for more important operations and urgencies.
Milli-Q® water purifications systems provide simplified operations, maintenance and support for a more efficient working day. Combined with our deep technical expertise and MyMilli-Q™ digital services, you’ll be able to achieve greater overall lab productivity.
- Care-free system operation
- Achieve more water system uptime with MyMilli-Q™ Remote Care
- Easy cartridge changes that can be managed in-house
- Walk-away filling with Volumetric Dispense mode on POD dispensers
- Only once-a-year maintenance by a service professional
- Reactive & knowledgeable Milli-Q® Services team in every geography
- Automatic notifications when care is needed with MyMilli-Q™ Remote Care
- Simplified online contract management

Past and future maintenance actions are clearly displayed on the interactive MyMilli-Q™ Event Traceability tool.
Improve Quality Management & Data Traceability
Never miss a data point again. Milli-Q® water purification systems automatically record all water quality parameters, volumes and system events directly in the system. Events include alerts, setting modifications, purification cartridge replacements, service activities, and more. This is especially critical for clinical laboratories, as water quality must be documented for labs seeking to conform to CLSI® and requiring accreditation (or reaccreditation) to the ISO 15189:2022 standard supported by CAP 15189SM accreditation.
- E-record archiving facilitates accreditation as all data generated are automatically stored in the system memory.
- RFID technology ensures automatic traceability of purification cartridges and prevents the insertion of an incorrect consumable.
- The Event Traceability tool‡ makes it easy to view events by type and over a specified timeline and supports planning for future maintenance.
- Water quality parameters (resistivity, temperature, and TOC) are rapidly searchable and graphable over any specified timeline‡.
‡For systems connected to MyMilli-Q™ Remote Care.
Related Resources
- Brochure: Water Purification Solutions for Clinical Laboratories
Discover water systems that drive consistency, efficiency and productivity.
- White Paper: Guide for an Efficient Clinical Laboratory
Learn best practices for efficient water purification system operation and management .
- Brochure: Milli-Q® CLX 7000 Connected, CLRW Clinical Water Systems
The water purification solution must reliably provide your clinical analyzers with consistent water quality that meets CLRW standards of the CLSI®.
- Article: Water for Clinical Chemistry
Explore how high-quality water is essential for accurate clinical chemistry assays and complying with norms and guidelines (e.g. CLSI).
- Article: Ultrapure Water for LC-MS Biomedical Analyses
This study assesses the suitability of ultrapure water from a Milli-Q® water system for sensitive LC-MS analyses of hormones.
- Article: Assessing the Level of Bacteria in Purified Water
Critical points for laboratories to consider when assessing bacteria levels in purified water and attempting to optimize the microbiology process.
- Datasheet: CLX Microbiological Sampling Procedures
How to acquire and prepare high-purity water samples from Milli-Q® CLX water systems for microbiological analysis.
- Article: Water for Histology Staining
Reliable histology staining requires consistent quality pure water. See examples of GMS and H&E stains performed with various water contaminants and comparing tap vs Type 2 water.
- Article: Water for DNA Sequencing
Learn how water impurities can impact DNA sequencing from Sanger to Next-Gen, single cell sequencing and beyond. High purity water purified using ultrafiltration is optimal for successful sequencing and related techniques.
To continue reading please sign in or create an account.
Don't Have An Account?