Novabiochem® Fmoc-Amino Acids
Introduction
For peptide chemists, the efficiency of each synthetic step is critically important in determining the quality of the final peptide product. Equally important, yet often underestimated, is the influence of starting material purity on synthetic outcomes. Even marginal improvements in the chemical purity of Fmoc-protected amino acids can yield significant increases in overall peptide yield, as demonstrated in Figure 1. To enhance reproducibility and reliability, the 20 proteinogenic Novabiochem® Fmoc-amino acids are produced under advanced specifications, thereby providing researchers with greater assurance in achieving consistent, high-quality synthesis results.
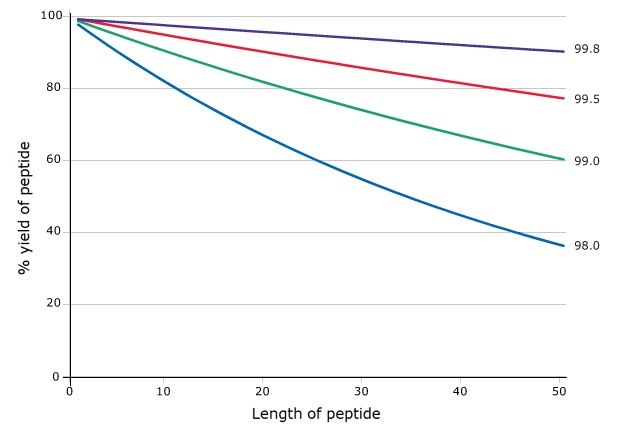
Figure 1.Effect of building block purity on step-wise peptide yield.
Stringent Specification Parameters of Fmoc-amino acids
- Free amino acid content ≤0.2% prevents double insertion during chain elongation and enhances long-term stability of the protected amino acid.
- HPLC purity ≥99% and enantiomeric purity ≥99.8% ensure the synthesis of peptides with high chemical and stereochemical integrity, supporting reproducible quality.
- Acetate content ≤0.02% minimizes the accumulation of truncated peptide sequences caused by chain-terminating side reactions.
- Ethyl acetate content ≤0.5% reduces the risk of acetic acid formation during extended storage and preserves reagent stability.
- Amino acid–derived impurities (β-alanyl species, dipeptides, and side-chain deprotected Fmoc-amino acids) ≤0.1% ensure cleaner synthesis profiles and improved reproducibility.
Benefits of Purer Fmoc-amino acids
- Increased yields and optimized purification: Enhanced amino acid purity improves overall peptide quality, thereby reducing the complexity of downstream purification.
- Improved scalability and regulatory compliance: Consistent impurity profiles support reproducible outcomes and facilitate smoother transitions to GMP-regulated manufacturing.
- Reduced synthesis failures and troubleshooting burden: The use of highly defined building blocks minimizes variability, providing greater reliability and confidence in synthetic results.
Hidden Limitations of Apparent HPLC Purity
During the synthesis of Fmoc-protected amino acids, predictable by-products such as racemized enantiomers, residual protecting-group fragments, and partially deprotected side chains may be generated. Consequently, a high reported HPLC purity does not necessarily indicate the absence of such impurities, which can diminish coupling efficiency, lower peptide yields, and introduce undesired by-products. Furthermore, conventional HPLC techniques cannot resolve enantiomeric species, thereby leaving trace D-enantiomeric contaminants undetected. Even at low concentrations, these impurities may compromise peptide conformation, bioactivity, and overall reproducibility.
Thus, reliable assessment of Fmoc quality requires more than apparent HPLC purity values. Rigorous evaluation of enantiomeric excess and comprehensive impurity profiling are essential to ensure reproducible synthesis and compliance in regulated manufacturing environments.
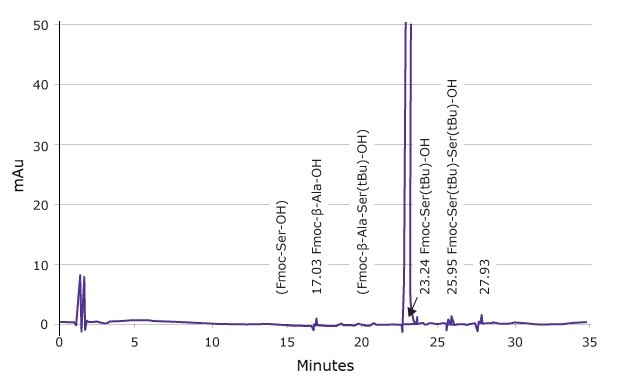
Figure 2.Actual HPLC profile of Fmoc-Ser(tBu)-OH, showing amino acid impurities clearly identified
For Novabiochem® Fmoc-amino acids, impurity characterization is performed using defined reference standards in combination with highly optimized HPLC methodologies. This strategy enables unambiguous separation of impurities from the parent compound and permits their accurate quantification.
Additional Impurities Beyond Enantiomers
- Acetic acid: Even trace concentrations of acetic acid can induce chain termination events during peptide synthesis. To mitigate this risk, all 20 proteinogenic Novabiochem® Fmoc-amino acids are specified to contain less than 0.02% acetate.
- Free amino acids: Residual unprotected amino acids can destabilize the Fmoc group and reduce overall coupling efficiency. To ensure reliability, quantitative GC-based analysis is applied, and all Novabiochem® Fmoc-amino acids are consistently specified to contain less than 0.2% free amino acids.
Sources of Impurities in Fmoc-Amino Acids
Impurities in Fmoc-protected amino acids arise predominantly from predictable side reactions during attachment of the Fmoc group to the amino acid.
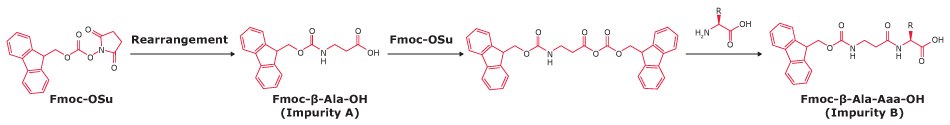
Dipeptides
Reaction of the Fmoc attachment reagent with an already formed Fmoc-amino acid generates dipeptide impurities. These can cause double insertion of the target amino acid during peptide synthesis.
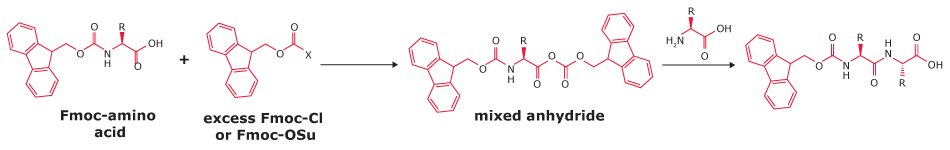
β-Alanyl Impurities
Ring opening and rearrangement of Fmoc-OSu, the reagent used for Fmoc introduction, produces β-alanyl impurities. These can lead to insertion of an additional β-alanine residue or substitution of the target amino acid with β-alanine.
Free Amino Acids
Incomplete reaction of the amino acid with the Fmoc reagent results in residual free amino acids. These can cause multiple insertions of the target amino acid and promote autocatalytic cleavage of the Fmoc group during storage, reducing reagent stability.
Acetic Acid Contamination
Acetic acid contamination in Fmoc-amino acids originates primarily from the use of ethyl acetate during preparation and crystallization. Hydrolysis of ethyl acetate produces acetic acid, while residual solvent can undergo slow transesterification with Fmoc-amino acids during storage, generating additional acetic acid over time.
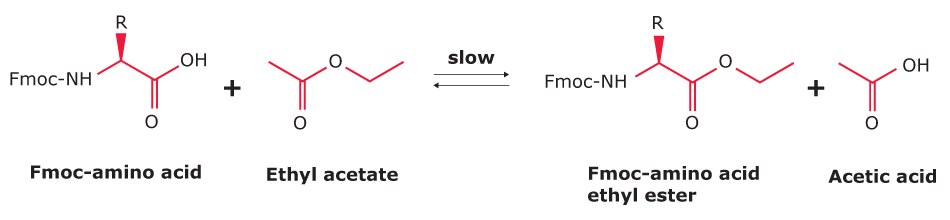
Even at trace levels, acetic acid has a disproportionate effect on peptide synthesis due to its low molecular mass and high reactivity. For example, just 0.1% acetic acid in Fmoc-Arg(Pbf)-OH corresponds to ~1 mol% contamination. Under synthesis conditions with a 5-fold reagent excess, this can lead to up to 5% chain termination per cycle. In peptides with multiple arginine residues, the cumulative impact can be as high as 15% chain termination.
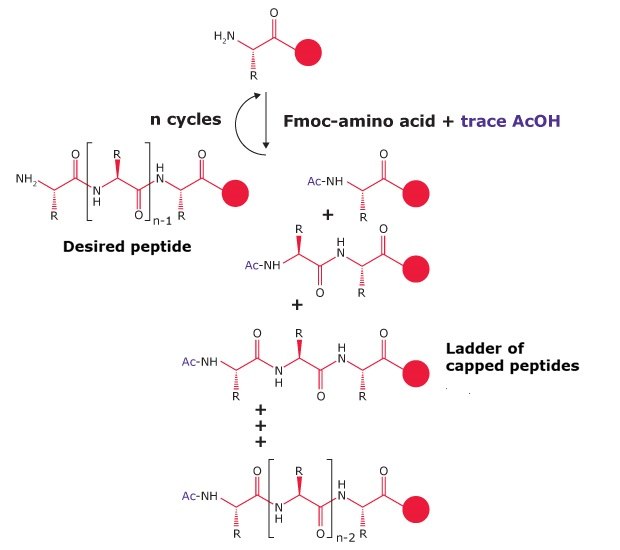
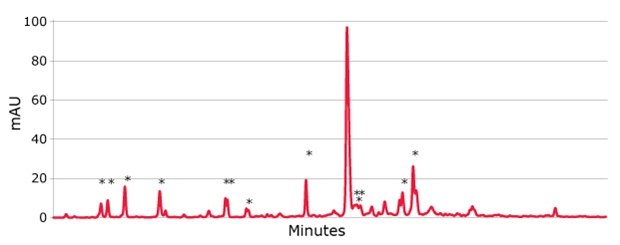
Figure 3.The effect of just 0.1% acetic contamination on the synthesis of a 21 mer.
truncated by-products are marked by an asterix
Detecting acetic acid in Fmoc-amino acids is particularly hard as it is invisible to HPLC and cannot be identified in trace amounts by 1H NMR. To address this, specialized analytical methods have been developed to quantify acetic acid reliably.
Both acetate and ethyl acetate contents are therefore stringently specified in all 20 standard Novabiochem® Fmoc-amino acids, ensuring reliability and long-term stability in peptide synthesis.
To continue reading please sign in or create an account.
Don't Have An Account?