Cell Culture Media Fingerprinting: Ensuring Identity, Consistency, and Quality
Cell culture media (CCM) are complex formulations that can consist of more than 60 components, including amino acids, vitamins, nucleosides, carbohydrates, trace elements, and salts. Each component of the formulation plays an important role in cell growth, culture performance, and the quality of the therapeutic proteins being produced. Because even small variations in the composition of a formulation can negatively impact process performance, yields, and product quality, ensuring media identity, consistency, and quality is essential.
This page describes the development of a comprehensive CCM fingerprinting technique that can rapidly and cost-effectively identify the presence and concentration of more than 100 media formulation components. By applying this advanced analytical technique to our CCM formulations, we reduce the risk of variability and enable customers to more effectively meet stringent regulatory requirements related to raw materials used in drug production.
CCM Fingerprinting
Identifying and characterizing the components in a CCM formulation, a process called fingerprinting, supports batch release, media development and optimization, as well as troubleshooting.
Combining multiple analytical techniques for CCM fingerprinting, such as Raman and IR spectroscopy, poses challenges due to varied protocols, sample preparation requirements, and instrumentation limitations. In addition, these methods often fall short of delivering detailed compositional profiles of CCM. Using multiple analytical techniques for CCM fingerprinting is also time-consuming and resource-intensive, making it an inefficient and costly approach.
Given these challenges, there is a clear need for an advanced analytical tool capable of providing both qualitative and quantitative insights into CCM components. Industry groups like the BioPhorum Operations Group (BPOG) have recognized this gap and continue to emphasize the need for more efficient media identity testing.1
A more powerful, streamlined analytical approach to CCM fingerprinting would offer drug developers significant advantages, including simplified verification of incoming media batches and improved consistency. It would also support faster, more confident decision-making across media development, manufacturing, and quality control.
A New Approach to Comprehensive Media Characterization: LC-MS Fingerprinting
Because CCM contains diverse compounds spanning multiple chemical classes and concentrations, from high-abundance salts to trace-level vitamins, our organization identified liquid chromatography coupled with mass spectrometry (LC-MS) as being well-suited for comprehensive characterization of CCM. The sensitivity, selectivity, and broad dynamic range of this method make it ideal for simultaneously detecting and quantifying a wide array of analytes of different concentrations in media.
To develop a LC-MS fingerprinting method, a master list of over 500 compounds commonly used in CCM was identified. From this, a focused panel of analytes based on two key criteria was selected: their widespread inclusion in media formulations and their compatibility with MS-based detection. Each analyte underwent a rigorous evaluation process, assessing properties such as ionization efficiency, chromatographic retention, MS/MS fragmentation patterns, and signal quality in both positive and negative ionization modes. Detection limits and known degradation pathways were also considered to ensure reliable quantification.
The result of this initiative is a robust, validated method capable of measuring over 100 distinct CCM components, including amino acids, vitamins, nucleosides, peptides, buffers, and polyamines. This LC-MS platform supports comprehensive media identity testing post-manufacturing, offering insight into lot-to-lot consistency, intra-lot homogeneity, and potential formulation deviations before use by customers. Sample preparation is straightforward, requiring only dilution with no need for derivatization.
Accurate Identification of Media Components with LC-MS Fingerprinting
As shown in Figure 1, LC-MS fingerprinting significantly enhances visibility into media composition. Starting with a standard CCM formulation containing 81 components, traditional amino acid and vitamin testing provides coverage for only about 42% of the formulation. Applying the LC-MS-based fingerprinting method alone increases that coverage to 74%. The most comprehensive view is created when combining CCM fingerprinting and trace element (TE) analysis; here, the total component coverage reaches 91%. In some cases, 100% component coverage can be achieved when combining LC-MS fingerprinting and TE analysis. This integrated approach delivers the most complete understanding of media composition, supporting better control, consistency, and performance.
Detected components per applied method
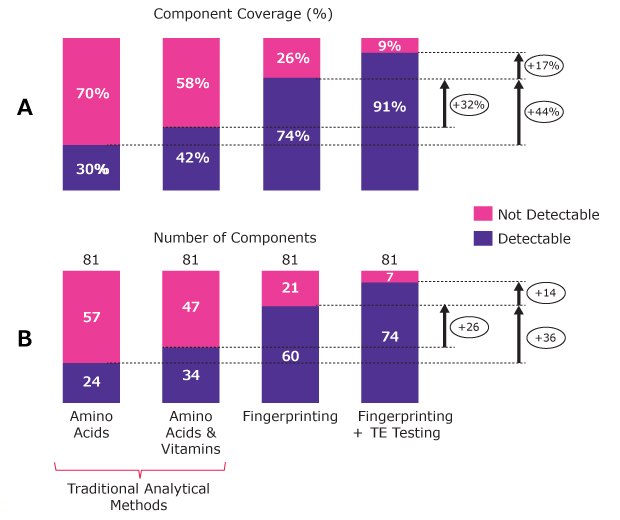
Figure 1.Representative analysis of a cell culture medium with different analytical techniques showing the component coverage (A) and number of components detected (B) for a medium formulation containing 81 components. Use of LC-MS fingerprinting increases the identification of media component coverage to 74%, and when combined with trace element analysis, the percentage rises to 90%.
Advantages for Characterization and Investigation
In addition to supporting media identity testing, the LC-MS method generates a wealth of data and insights relevant to broader applications in CCM, including root cause investigations and the design of custom formulations tailored to specific process requirements. This method has been used to support the development and optimization of custom media formulations for customers, resulting in improved cell growth and increased product titers (Figure 2).
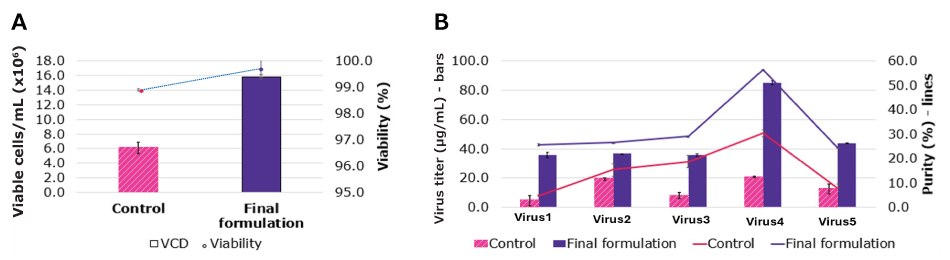
Figure 2.Cell culture growth profile (A) and titer (B) of control (original) and final (optimized) formulation. The medium was optimized using data from LC-MS fingerprinting. The optimized formulation showed over 160% increase in viable cell density and about 100% increase in titer for four viruses and over 300% titer increase for one virus.
Conclusion
As biologics manufacturing continues to evolve in complexity, establishing true compositional identity of CCM is becoming increasingly vital, not only for ensuring quality and consistency, but also for meeting stringent regulatory expectations.
Traditionally, CCM identification has relied on techniques like Raman and IR spectroscopy. While useful for some applications, these methods fall short in delivering the level of compositional detail required to guarantee batch performance and reproducibility.
Our LC-MS-based fingerprinting platform bridges this critical gap. It offers a major advancement in the accurate identification and characterization of CCM formulations, ensuring consistency, reliability, and quality for end users. With a single analytical protocol, this method quantifies more than 100 media components across diverse chemical classes, from abundant salts to trace-level vitamins and degradation products. It combines high sensitivity with selectivity, distinguishing closely eluting compounds while requiring minimal sample preparation and delivering fast turnaround.
As a powerful tool for media characterization, this platform enables proactive verification of lot-to-lot consistency, intra-lot uniformity, and overall process stability. In doing so, it helps reduce the risk of costly deviations and gives biomanufacturers the insight and confidence needed to optimize performance and maintain product quality.
Would you like to discuss this technology or request more information?
References
To continue reading please sign in or create an account.
Don't Have An Account?