Atomic Spectroscopy Overview
Atomic spectroscopy uses the electromagnetic radiation or mass spectrum of a sample to determine elemental composition. The wavelength of energy absorbed or emitted by atoms is characteristic to each element and can be used for element identification and quantification.
Analytical techniques based on atomic spectroscopy are widely used in environmental chemistry, geology and soil science, mining and metallurgy, food sciences, and medicine.
Featured Categories
Enhance inorganic trace analysis with our certified AAS and ICP standards. NIST-traceable solutions available.
Browse through our selection of reference materials of inorganic elemental impurity mixes as per ICHQ3D guidelines for your ICP or AAS based tests of drug products in pharmaceutical analysis.
Uncover a wide acid range: Supelco® for analysis, Sigma-Aldrich® for labs, SAFC® for biopharma. Tailor solutions for varied needs.
Milli-Q® systems offer innovative water purification technologies engineered to support lab research needs, sustainability goals, and other major requirements.
Atomic absorption spectroscopy (AAS)
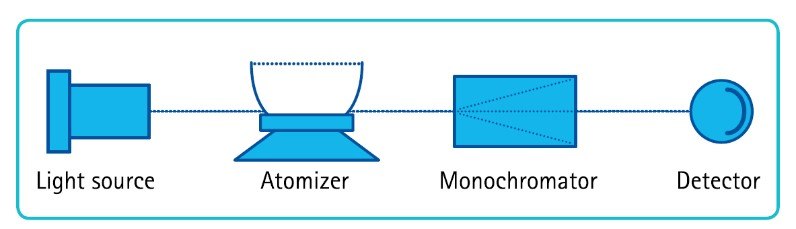
Atomic absorption spectroscopy (AAS) works by measuring the amount of UV/visible light energy absorbed by an element. The wavelength of light absorbed corresponds to the energy needed to promote its electrons from the ground state to a higher energy level. The amount of energy absorbed in this excitation process is proportional to the concentration of the element in the sample.
Flame atomic absorption spectroscopy (FAA)
Flame atomic absorption spectroscopy (FAA) involves vaporization and thermal atomization of a liquid sample by a flame. In this technique, a sample solution is aspirated and sprayed as a fine aerosol into a chamber to combine with fuel and oxidant gases. The resulting mixture is then carried to the burner head, where combustion and sample atomization occurs.
Graphite furnace atomic absorption spectroscopy (GFAA)
Graphite furnace atomic absorption spectroscopy (GFAA) is the most advanced and sensitive technique to assess atomic absorption. With a graphite furnace atomizer, the atoms are retained in the optical path for a slightly longer time compared with flame atomization, resulting in lower detection limits and sensitivity in the parts per billion (ppb) range.
Inductively coupled plasma optical emission spectroscopy (ICP-OES)
Inductively coupled plasma optical emission spectroscopy (ICP-OES) measures the light emitted by excited electrons of an element while returning to their stable ground state. The sample is introduced into an argon plasma and high temperature excites the atom’s electrons to higher energy levels. The element is identified by the characteristic wavelength of the light emitted as its electrons return to ground state. The intensity of the light emitted is related to the concentration of the element in the sample.
Inductively coupled plasma-mass spectrometry (ICP-MS)
Inductively coupled plasma mass spectrometry (ICP-MS) is a type of mass spectrometry used for the highly sensitive quantification of various metals and non-metals in the concentration range of below 1 part per trillion (ppt). ICP-MS analyzes elements by their separation in a magnetic field as per their mass to charge (m/z) ratio.
X-ray fluorescence (XRF) spectrometry
X-ray fluorescence (XRF) spectrometry detects elemental composition by measuring the wavelength and intensity of X-rays emitted by energized atoms in a sample. In this method, a beam of short wavelength x-rays strikes the sample and dislodges innermost shell electrons of the atom, forming a vacant site or “hole”. This causes the atom to rearrange its electronic arrangement with an electron from a higher energy shell jumping to occupy the newly created vacancy and emitting characteristic X-ray light during the process. The X-rays emitted by the atoms during the process of fluorescence are detected and used for sample identification and quantitation.
Visit our document search for data sheets, certificates and technical documentation.
Related Articles
- Elemental impurities in pharmaceutical drugs present a risk to human health and are regulated. Milli-Q® ultrapure water purification systems comply with the requirements for trace element analyses.
- 의약품 내 원소 불순물은 개발 및 생산의 모든 단계에서 모니터링 및 관리되어야 합니다. Milli-Q® 정제수 시스템으로 생산된 초순수는 ICP-MS 및 ICP-OES 미량 원소 분석에 권장됩니다.
- Control elemental impurities in drug products with analytical methods and materials to ensure patient safety.
- 환경 시료의 미량 원소 분석 시 신뢰할 수 있는 결과를 내기 위해 Milli-Q® 초순수가 얼마나 엄격한 순도 요구조건을 충족하고 있는지 설명한 데이터를 확인해 보십시오.
- 최적의 물 시스템을 찾기 위한 필수 단계. 품질부터 친환경 기술 및 공간 절약 옵션까지.
- See All (12)
Related Protocols
- Milli-Q® SQ 2Series 실험용 정제수 시스템에 대한 지침 영상을 보고 사용자 설명서를 다운로드하십시오. 즉시 설치하여 사용할 수 있습니다. 시스템, 액세서리, 카트리지 및 서비스를 온라인으로 구매하십시오.
Find More Articles and Protocols
How Can We Help
In case of any questions, please submit a customer support request
or talk to our customer service team:
Email custserv@sial.com
or call +1 (800) 244-1173
Additional Support
- Chromatogram Search
Use the Chromatogram Search to identify unknown compounds in your sample.
- 계산기 및 앱
웹 툴박스 - 분석 화학, 생명과학, 화학 합성 및 재료 과학을 위한 과학 연구 도구 및 리소스.
- Customer Support Request
씨그마 알드리치의 주문, 제품, 계정 및 웹사이트에 대해 궁금하신 부분을 확인할 수 있습니다
- FAQ
Explore our Frequently Asked Questions for answers to commonly asked questions about our products and services.
계속 읽으시려면 로그인하거나 계정을 생성하세요.
계정이 없으십니까?