Liquid Formulation Solubility Enhancement
For an active pharmaceutical ingredient (API) to achieve its intended therapeutic effect, it must be sufficiently soluble to ensure proper absorption and bioavailability in the body. Poor solubility of an API, whether in a solid or liquid dosage form, can significantly reduce bioavailability, necessitating the use of formulation strategies to overcome this limitation. Many promising therapeutics fail to achieve market approval due to insufficient solubility.
If you are interested in learning more about the basics of solubility, please read our dedicated technical article here.
Solubility Challenges of Injectable Formulations
It is estimated that 90% of all new molecular entities are poorly soluble; a significant number of these drugs are formulated as injectables (Figure 1). Liquid oral and parenteral formulations present unique challenges related to solubility. If these challenges are not addressed, the success of the final drug product can be jeopardized. Sufficient solubility is essential to ensure the API remains dissolved throughout the shelf-life of the drug product and that the solution remains free of visible particles. Achieving the necessary level of solubility can be particularly difficult when dealing with high-concentrated liquid formulations.
NME Dosage form of Newly Approved Drugs (NMEs)
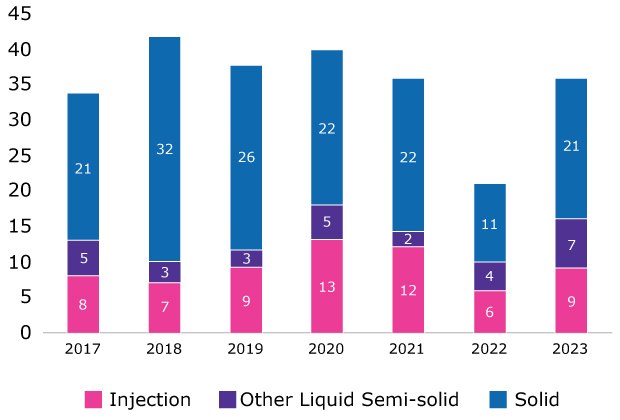
Figure 1.FDA database information demonstrates that a significant portion of new molecular entity dosage forms and marketed drugs are injectables.1
Formulation-Based Approaches to Enhance API Solubility
Developing effective liquid drug formulations requires strategies that enhance the solubility of the API. Various excipients and formulation approaches can improve bioavailability by increasing the API's solubility in aqueous solutions. Common strategies include the use of cyclodextrins for molecular encapsulation, polyethylene glycol (PEG) as a solubilizing agent, meglumine as counterion or functional excipient, as well as co-solvents or pH adjusters. Each of these approaches plays a critical role in optimizing drug performance and ensuring consistent therapeutic outcomes.
Cyclodextrins
General Information on Cyclodextrins
Cyclodextrins are cyclic oligosaccharides derived from natural starch. Their structure consists of α-1,4-D-glucopyranoside units arranged in the form of a hollow cone, featuring a hydrophilic exterior and a hydrophobic cavity (Figure 2). This structure allows cyclodextrins to interact with hydrophobic, poorly water-soluble APIs, forming reversible inclusion complexes that facilitate the release of the API upon administration.
Cyclodextrins are classified as α, β, or γ depending on the number of glucopyranoside units, with α containing six units, β containing seven, and γ containing eight. Derivatization with functional groups, such as hydroxypropyl and sulfobutylether, significantly enhances their solubility, making these derivates the preferred choice for pharmaceutical formulations: Recent years have shown a notable increase in drug products containing 2-hydroxypropyl-β-cyclodextrin (HP-β-CD) and sulfobutylether-β-cyclodextrin sodium salt (SBE-β-CD), with approximately 50% of HP-β-CD and over 90% of SBE-β-CD containing parental drug products. Well-known APIs formulated with these derivatives include remdesivir, voriconazole, and itraconazole.
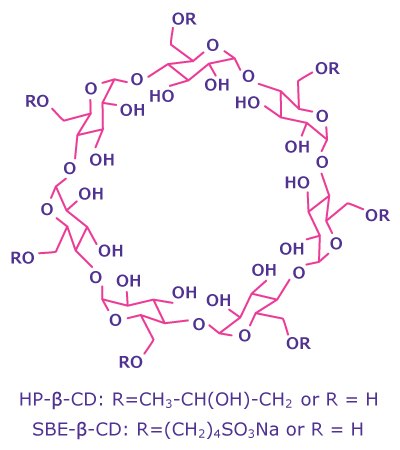
Figure 2.Molecular structure of β-cyclodextrin derivates.
Solubility Enhancement with Cyclodextrins
The unique characteristics of cyclodextrins enable their use for increasing solubility and stability, protecting APIs against chemical degradation, and reducing aggregation.
The solubility of the poorly soluble model API ibuprofen can be significantly increased when complexed with HP-β-CD. At an API:cyclodextrin ratio of 1:10, ibuprofen solubility was approximately 80 times higher than that of the pure API (Figure 3).
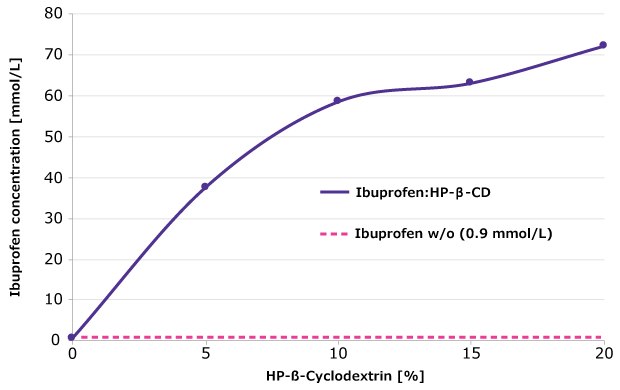
Figure 3.Solubility of ibuprofen as a function of HP-β-CD concentration.
Experimental conditions: Incubation of ibuprofen with different concentrations of HP-β-CD (0–20 %) at pH 5.0 (50 mmol/L acetate buffer) for 24 h at 25 °C; centrifugation at 16,000 rpm. Ibuprofen concentration in the supernatant determined by HPLC.
SBE-β-CD can further enhance the solubility of certain APIs beyond that achieved with HP-β-CD. The longer hydrophobic chain of sulfobutyl ether in SBE-β-CD increases the hydrophobic region within the cavity, allowing for a more effective complexation. For instance, the complexation of model API itraconazole with SBE-β-CD was shown to enhance the solubility up to 125-fold (even in a non-optimized formulation) compared to the pure API (Figure 4).
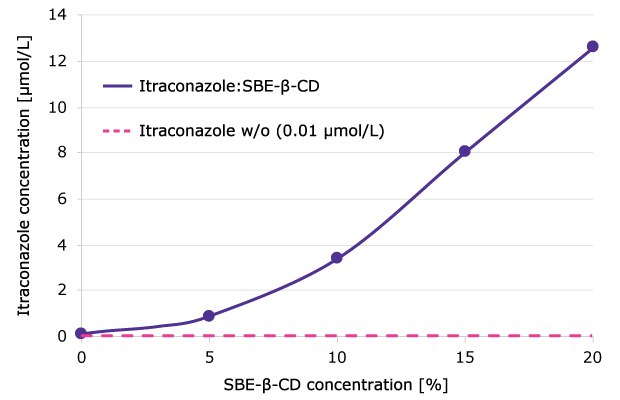
Figure 4.Solubility of itraconazole as a function of SBE-β-CD concentration.
Experimental conditions: Incubation of itraconazole with different concentrations of SBE-β-CD (0–20 %; API:Cyclodextrin ratio of up to 1:10) at pH 5.0 (50 mmol/L acetate buffer) for 24 h at 25 °C; centrifugation at 16,000 rpm. Itraconazole concentration in the supernatant was determined by HPLC.
Improving API Stability with Cyclodextrins
In addition to enhancing solubility, cyclodextrins support improved API stability by protecting APIs against hydrolysis or oxidation. For biologic APIs, the shielding effect of cyclodextrins increases resistance to mechanical and temperature stress, thereby reducing aggregation and turbidity formation after shear and shaking stress and helping to maintain higher monomer content. Furthermore, HP-β-CD has also been shown to be an effective alternative to polysorbates and other surfactants, helping to mitigate surfactant-related oxidative degradation impurities.2
Safety Considerations of Cyclodextrins
Cyclodextrins also minimize or prevent gastrointestinal and ocular irritation and can alleviate undesired characteristics of APIs, such as unpleasant taste, odor, and toxicity as a result of their shielding effect.
Both HP-β-CD and SBE-β-CD have established safety profiles, having been used in pharmaceutical products for decades. They are listed in the FDA Inactive Ingredient Database1, with numerous safety reports and dosing thresholds for various application routes further supporting their use.
Summary
Cyclodextrins are effective excipients that enhance API solubility and stability through the formation of inclusion complexes, with HP-β-CD and SBE-β-CD derivatives recognized for their safety and value in parenteral products (Table 1).
Polyethylene glycols
General Information on Polyethylene Glycols
PEGs are polyethers synthesized through the anionic polymerization of ethylene oxide with hydroxyl initiators (Figure 5). The mean molecular weight is determined by the polymer length and is included in the PEG name (e.g., PEG 200 has a mean molecular weight of 200 g/mol). The molecular weight of PEGs directly influences their physical form, characteristics, and application areas.
Figure 5.Chemical structure of polyethylene glycol
Solubility Enhancement and Other Applications of Polyethylene Glycols
PEGs serve as solubilizers, lubricants, binders, and consistency enhancers. Their high polarity makes them extremely hydrophilic, which allows them to increase the solubility of a broad range of APIs. PEGs are fully miscible with aqueous fluids and effectively dissolve many poorly water-soluble compounds. Drugs with limited aqueous solubility often suffer from poor bioavailability and significant variability in absorption; however, when administered as solutions or suspensions in PEGs, these have demonstrated markedly improved bioavailability and reduced inter-subject variability in plasma concentrations.3
More than 6,000 marketed drug products contain PEGs, including small-molecule drug formulations as well as topical, parenteral, and other applications. Many formulations incorporate two or more PEGs to achieve optimized characteristics.
PEGs are utilized in various FDA-approved dosage forms, with their distinct molecular weights rendering each grade particularly suitable for specific applications. Notably, PEG 300, 400, 3350, and 4000 are employed in FDA-approved parenteral drugs. (Table 2).1
Low molecular weight PEGs, such as PEG 200 to PEG 400, are liquids that are miscible in water at nearly any ratio. These liquid PEGs are widely used as solubilizers in dosage forms like drops or capsule fillings, as lubricants in eye drops, and as emulsion stabilizers in creams and ointments. Additionally, PEGs are non-volatile, offering significant advantages over traditional solvents by being easier and safer to handle while preventing vaporization during storage.
PEG 1000 and 1500 are pasty or soft solids, while PEG 3000 and higher molecular weights are solids. As solid PEGs have relatively low melting points, they are ideal excipients for hot melt extrusion, supporting solubility enhancement also through the formation of amorphous solid dispersions in solid formulation applications.
Safety Considerations of Polyethylene Glycols
PEGs are generally recognized as safe (GRAS) by the FDA, with extensive use in pharmaceutical formulations. Their low toxicity and favorable safety profiles have been well-documented in various studies, supporting their application across a wide range of dosage forms. Additionally, PEGs are non-volatile, offering significant advantages over traditional solvents by being easier and safer to handle while preventing vaporization during storage.
Summary: Polyethylene glycols are versatile excipients known for their solubilizing properties and their ability to enhance emulsion and suspension stability, making them essential in various liquid formulations.
Meglumine
General Information on Meglumine
Meglumine, or methylglucamine, is a sorbitol derivative with a terminal amino group (Figure 6) that acts as a counterion to APIs, forming a better soluble API salt, or as a functional excipient. Its pKa value of 9.60, makes it well applicable for weakly acidic APIs (pKa ≤ 6.0). In addition to enhancing solubility, meglumine is also used to improve API stability, and adjust pH values in both solid and liquid formulations.
Figure 6.Chemical structure of meglumine.
Solubility Enhancement with Meglumine
The ability of meglumine to enhance the solubility of APIs in liquid formulations has been demonstrated for a variety of APIs. Figure 7 summarizes the results of solubility with poorly soluble model API ibuprofen: API solubility increased with increasing meglumine concentrations. Especially relevant for liquid formulations, the solutions of ibuprofen were stable at high API concentrations (up to 100 mg/mL), stable after steam sterilization (storage stability studies conducted for 3 months at accelerated conditions), and filterable using various commonly used filter types. Consequently, these results confirm the suitability of meglumine for liquid formations.
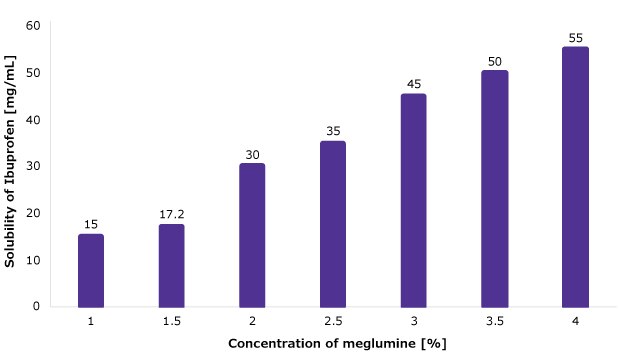
Figure 7.Solubility of ibuprofen when combined with increasing concentrations of meglumine.
Safety Considerations of Meglumine
Meglumine is an FDA approved excipient in pharmaceuticals and in contrast media, used for different dosage forms and administration routes, including parenteral formulations.
Summary: Meglumine serves as a counterion and functional excipient that enhances the solubility and stability of APIs, especially weakly acidic APIs, and is utilized across various pharmaceutical applications, including parenteral formulations.
Co-Solvents
Co-solvents are water-miscible solvents used to improve the solubility of injectable formulations. Commonly utilized co-solvents include glycerin, propylene glycol, polyethylene glycol 400, and ethanol.4 These co-solvents reduce the polarity of the solvent mixture, improving compatibility with non-polar drug molecules, thereby increasing their solubility.
pH Adjusters
To enhance the solubility of an API, pH adjusters such as sodium hydroxide (for acidic APIs) or hydrochloric acid (for basic APIs) can be employed to modify the solution pH. This approach converts the poorly soluble, neutral form of the API into a better soluble, ionized form by increasing the pH for weakly acidic APIs and decreasing it for weakly basic APIs (depending on the co-solvent used).
Conclusion
As the pharmaceutical industry grapples with the increasing number of poorly soluble APIs in development pipelines, solubility-enhancing functional excipients – such as cyclodextrin, PEG, and meglumine, but also co-solvents and pH adjusters – play a crucial role in advancing pharmaceutical formulation. Employing tailored formulation strategies not only improves solubility and bioavailability but also mitigates the risk of development failures, thereby increasing the likelihood of successfully bringing new therapies to market.
Looking ahead, the strategic use of these excipients will remain a key enabler in drug development, unlocking the potential of promising compounds that might otherwise never reach patients. By leveraging these solutions, the industry can effectively navigate formulation challenges and foster continued innovation, ultimately enhancing patient access to vital medications.
Need expert guidance? Reach out to our specialists to explore formulation solutions.
Related Products
References
Para seguir leyendo, inicie sesión o cree una cuenta.
¿No tiene una cuenta?