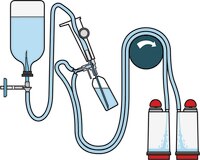
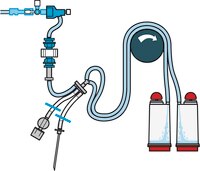
Steritest™ NEO Devices for Antibiotics and Products with Antimicrobial Activity
Red canister base with specific drain design. Low binding PVDF Durapore® membrane. This optimizes the rinsing of products that inhibit microbial growth. Canisters material: SAN
Más
Red canister base with specific drain design. Low binding PVDF Durapore® membrane. This optimizes the rinsing of products that inhibit microbial growth. Canisters material: SAN Menos
More>>
Less<<

Productos recomendados
Descripción
Especificaciones
Información para pedidos
Documentation
Referencias bibliográficas
| Visión general referencias | Pub Med ID |
|---|---|
| The Effect of Vaporous Phase Hydrogen Peroxide on Sterility Test Devices Roche Lentine, Kerry and Robert J. Keller; Pharmaceutical Technology Europe, February 2003: 31- 42. Pharmaceutical Technology Europe, February 2003: 31- 42. 2003 | The Effect of Vaporous Phase Hydrogen Peroxide on Sterility Test Devices
 |
Productos y aplicaciones relacionados
Familias de productos

Unidad Steritest EZ para disolventes, cremas, ungüentos e inyectables veterinariosGreen canister base with specific drain design. Low binding PVDF Durapore® membrane optimize the rinsing of products that inhibit microbial growth. Canisters material: polyamide polymer.Más información >> |

Steritest™ NEO Devices for Products Without Antimicrobial AgentsBlue canister base. Mixed esters of cellulose membrane. This membrane provides an optimal filtration flow rate for standard products. Canisters material: SAN (Styrene-Acrylo Nitrile)Más información >> |

Sterility TestingCountless configurationsMás información >> |

Steritest™ EZ Devices Double-PackedIncrease your test method reliability with our gamma sterilized sterility testing deviceMás información >> |

Steritest™ NEO AccessoriesFor faster, easier and safer sterility testing.Más información >> |
Productos relacionados por: Application Facete
| Sterility Testing |
Productos relacionados por: Brand Facete
| Steridilutor® |
| Steritest™ |
Categorías
| Industrial Microbiology > Sterility Testing > Membrane Filtration Sterility Test > Steritest™ NEO Devices for Antibiotics & Products with Antimicrobial Activity |
Liquids in Ampoules
TZHVAB210 & TZHVAB205 (double packed)
- Single needle for easy access to ampoules
- Separate vent needle
Liquids in Collapsible Bags
TZHVAB210 & TZHVAB205 (double packed)
- Single needle for easy access to collapsible bags
- Separate vent needle
Liquids in Large Vials
TZHVLV210 & TZHVLV205 (double packed)
- Vented double needle for large glass containers with septa
Liquids in Small Vials
TZHVSV210 & TZHVSV205 (double packed)
- Vented double needle for small vials with septa
Soluble Powders in Vials
TZHVDV210 & TZHVDV205 (double packed)
- Double needles for small vials with septa
- Vented double needle
- Simultaneously dissolves/dilutes the sample in sterile diluent and transfers the resulting solution to canisters
Soluble Powders in Ampoules
TZHVDA210
- Double needles for small vials with septa
- Vented double needle
- Simultaneously dissolves/dilutes the sample in sterile diluent and transfers the resulting solution to canisters
Medical Devices and Collapsible Bags
TZHVMD210
- Three adapters provided; male Luer, female Luer or single needle allow connection to a variety of test devices
- Separate vent needle
Powders and Superpotent Antibiotics
TZVC00010 + TZHVAB210 or TZHVAB205 (double packed)
- Tubing and needle assembly for antibiotics and products containing antimicrobial activity that require dilution or dissolution
- Aseptically connects the diluent or dissolution fluid to the product container for dilution
- Used for pooling superpotent antibiotics to reduce product membrane contact time when product is then filtered
- Contains vent with expansion chamber for optimized venting
- Diluted product subsequently filtered with Steritest™ NEO device (TZHVAB210)